A kind of fluoroalkylation method of steroid 17β-thiocarboxylic acid
A thiocarboxylic acid, fluoroalkylation technology, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of high content, ineffective control of reaction conditions, and complicated post-processing procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
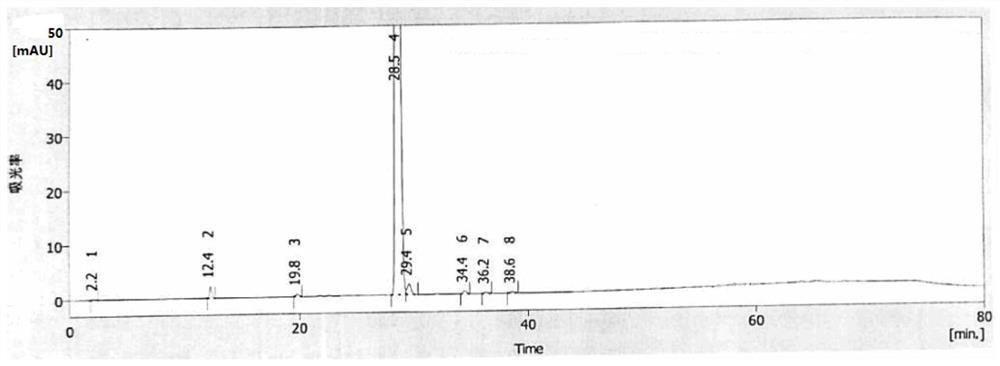
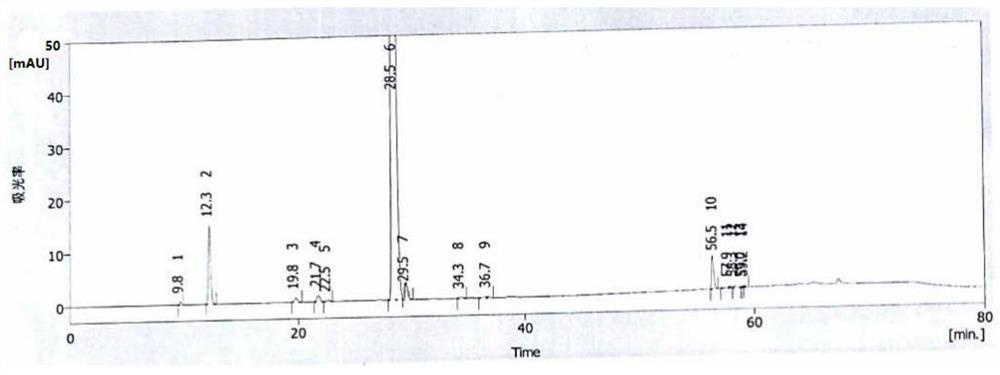
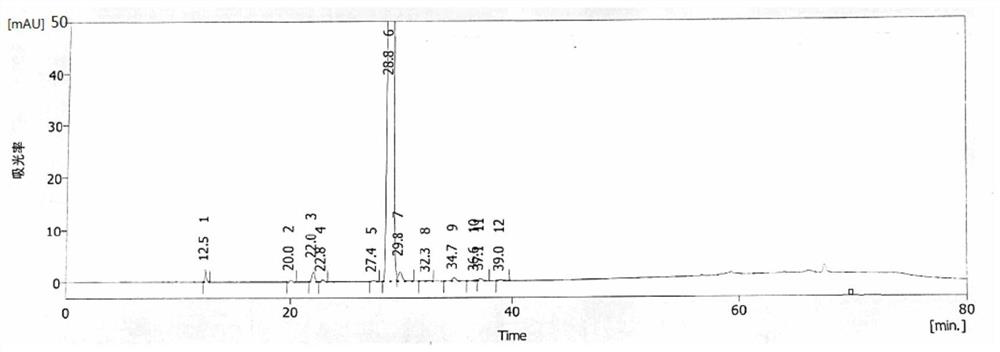
Image
Examples
Embodiment 1
[0063]
[0064] 50ml of dimethylacetamide and 10g of compound 1 were added to a four-neck reaction flask, cooled to -5°C, 2.9g of sodium sulfite was added, 2.0ml of bromofluoromethane was added dropwise, 500ml of purified water was added after the reaction was complete, a solid was precipitated, and the Filtration to obtain 10.4 g of compound 2 crude product, the detected compound 2 content is 98.8%, the mercapto oxide content is 0.38%, the haloalkylated impurity is 0.19%, and the disulfide compound is not detected. The obtained solid compound 2 crude product is added to absolute ethanol for recrystallization , suction filtration, and drying to obtain 10.1 g of compound 2, the content of which is 99.7%.
Embodiment 2
[0068]
Embodiment 2-1
[0070] Add 100ml of acetonitrile and 10g of compound 3 into a four-neck reaction flask, cool down to -15°C, add 4.0g of potassium carbonate and 1.0g of potassium sulfite, then dropwise add 2.0ml of fluoroiodomethane, add 500ml of purified water after complete reaction, and precipitate out Solid, suction filtration to obtain 10.3 g of compound 4 crude product, content 98.8%, mercapto oxide content 0.40%, haloalkylated impurities 0.21%, no disulfide compounds detected. The obtained solid compound 4 crude product was added to absolute ethanol for recrystallization, suction filtration, and dried to obtain 9.8 g of compound 4 fine product, with a content of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com