A kind of method for preparing obeticholic acid intermediate and obeticholic acid thereof
A technology of obeticholic acid and intermediates, applied in the field of drug synthesis, can solve problems such as difficult quality control and unfavorable initial quality control, and achieve the effects of easy tracking of reactions, reduced difficulty of refining, and high reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] carbonyl reduction reaction
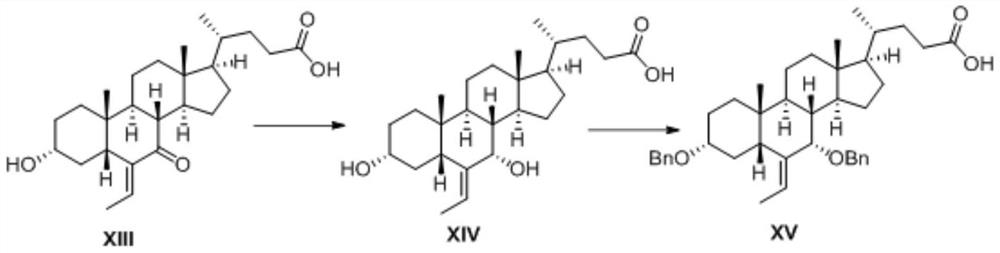
[0036] Preparation of Compound XIV
[0037]
[0038] In a 100ml round-bottomed flask, add 20ml of ethanol and 4.2g of compound XIII in sequence. After the solution is stirred and dissolved, add 0.95g of sodium borohydride in batches at a temperature of 10°C. The full reaction time is 8h. After the reaction is completed, add acetic acid Quench the reaction, add toluene and water, stir and separate layers, remove the water phase, wash the organic phase once with 5% acetic acid aqueous solution, dry, and spin dry to obtain the crude compound XIV, which is directly used in the next reaction, with a yield of 100%.
Embodiment 2
[0040] hydroxyl protection reaction
[0041] Preparation of Compound XV
[0042]
[0043] Add the crude compound XIV prepared in Example 1 to a 100ml round-bottomed flask and completely dissolve it in N,N-dimethylformamide solvent, then add 0.46g of potassium carbonate, and finally add 3.4g of benzyl bromide, heat at 40°C, and stir Reacted for 8 hours, cooled after the reaction, added toluene and 5% acetic acid aqueous solution, stirred and separated, removed the water phase, dried the organic phase and then spin-dried, recrystallized with toluene to obtain 5.5 g of intermediate XV, with a yield of 92%.
Embodiment 3
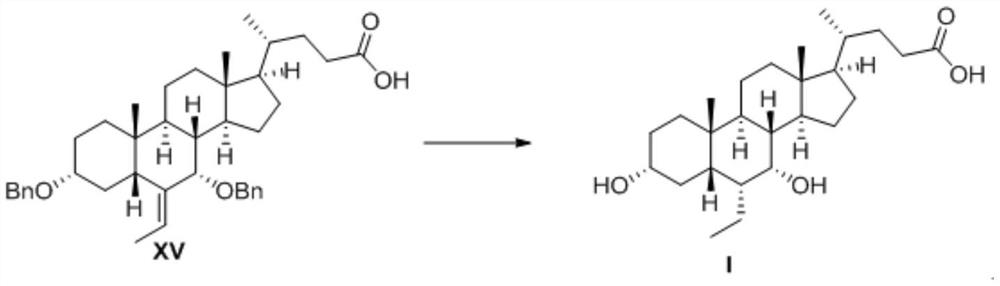
[0045] palladium carbon reduction reaction
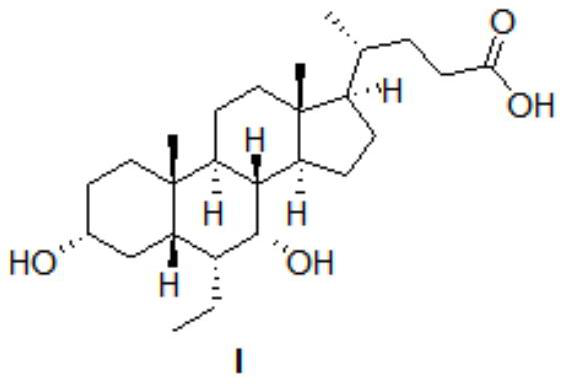
[0046] Preparation of obeticholic acid I
[0047]
[0048] 1) In a 100ml hydrogenation kettle, sequentially add 3.0 g of compound XV, 50ml of methanol, 0.005g of 10% Pd / C, 0.02g of sodium hydroxide and 100ml of water. After the mixture is evenly stirred, seal the hydrogenation kettle and use N 2 and H 2 Replace the air in the system, control the temperature at 20°C, control the hydrogen pressure at 0.5MPa, and react the mixture system for 2 hours under stirring;
[0049] 2) To terminate the reaction, use N in turn 2 and air displacement system H 2 ;
[0050] 3) After the mixture was filtered to separate Pd / C, the solvent was spun off to obtain a crude product of obeticholic acid, which was crystallized and purified with ethyl acetate to obtain 1.8 g of a refined product with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com