Latamoxef hydroxyl impurity preparation method
A technology of latamoxef hydroxy and oxyceph hydroxy, which is applied in the field of preparation of latamoxef hydroxy impurities, can solve the problems of few samples, poor impurity stability, and difficulty in obtaining pure samples, so as to achieve a large amount of sample acquisition and improve the overall quality level , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 50 g of tap water and 20 g of crystalline latamoxef sodium into a 250 ml reaction bottle, stir to dissolve, add 2.1 g of sodium hydroxide / 50 g of tap water solution, and react at a temperature of 40° C. for 5 hours. Cool down to room temperature, pass the reaction solution through the DM-2 resin column, and control the temperature of the resin column jacket at 2-10°C. After feeding the liquid, wash it with purified water. The bottom effluent is monitored with a capillary tube until there is 254nm fluorescence color development, and then the effluent is collected. 45-50 grams, after freeze-drying at the highest drying temperature of 5°C, 5.3 g of a light yellow solid was obtained, with a yield of 32.1%.
Embodiment 2
[0037] Add 70 g of tap water and 20 g of crystalline latamoxef sodium into a 250 ml reaction bottle, stir to dissolve, add 2.6 g of sodium hydroxide / 50 g of tap water solution, and react at a temperature of 45° C. for 5 hours. Cool down to room temperature, pass the reaction solution through the DM-2 resin column, and control the temperature of the resin column jacket at 2-10°C. After feeding the liquid, wash it with purified water. The bottom effluent is monitored with a capillary tube until there is 254nm fluorescence color development, and then the effluent is collected. 45-50 g, after lyophilization at the highest drying temperature of 5°C, 5.1 g of light yellow solid was obtained, with a yield of 30.9%.
[0038] Preparation by preparative chromatography 1 only about 2 mg of sample can be obtained for each separation, but by this method for preparation, the concentration of the feed liquid after passing through the macroporous resin can reach 10%-20%, and the direct freeze-...
Embodiment 3
[0039] Example 3 Sample Characterization
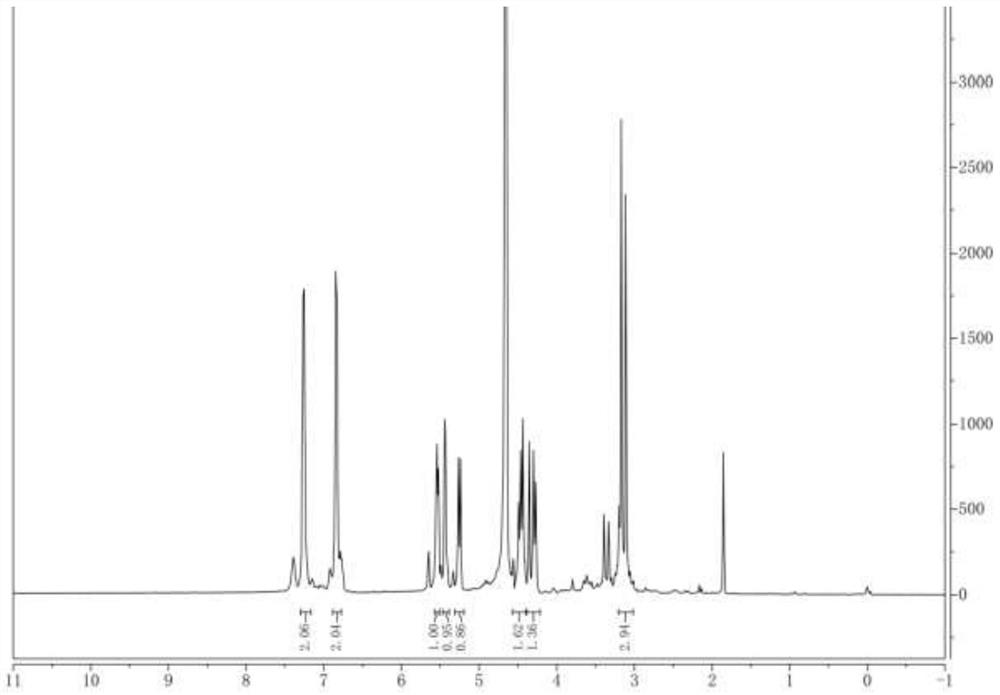
[0040] The Latamoxef hydroxyl impurity prepared by embodiment 2 is carried out to proton nuclear magnetic spectrum analysis, and analysis proton spectrogram is as follows figure 1 shown.
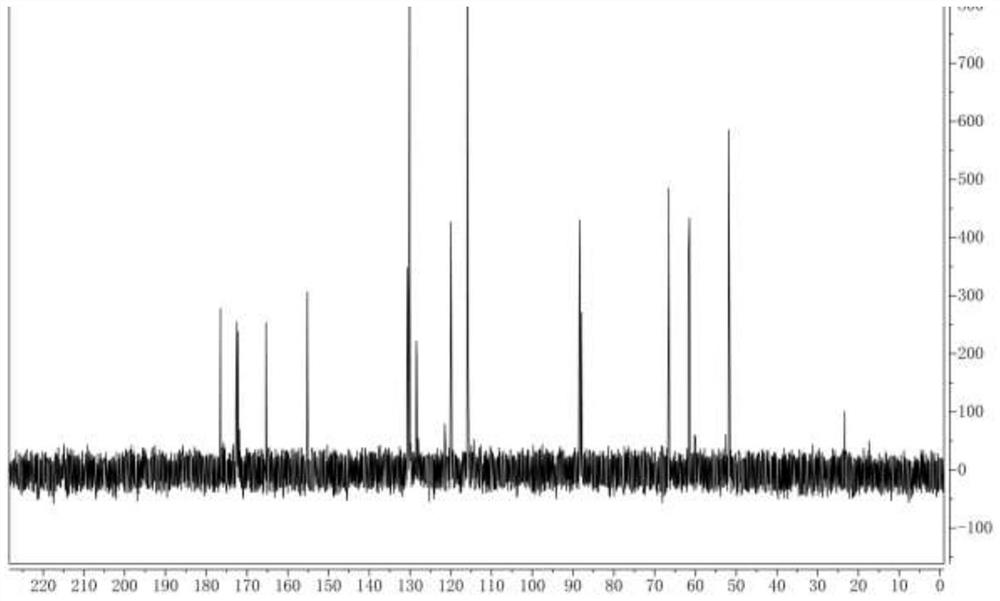
[0041] The Latamoxef hydroxyl impurity prepared in embodiment 2 is carried out carbon nuclear magnetic spectrum analysis, and analysis carbon spectrogram is as follows figure 2 shown.
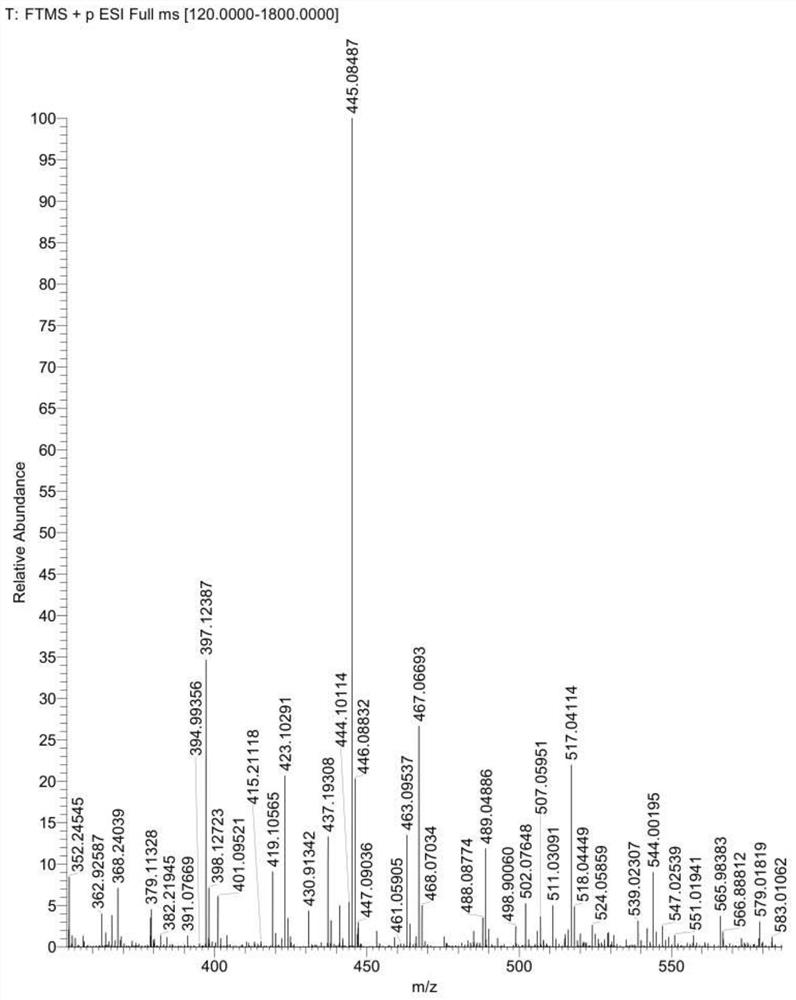
[0042] The Latamoxef hydroxyl impurity prepared by embodiment 2 is carried out to mass spectrometry, and the analysis mass spectrogram is as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com