Candesartan cilexetil and hydrochlorothiazide co-amorphous substance and preparation method thereof
A technology of candesartan cilexetil and hydrochlorothiazide, which is applied in the field of medicine, can solve the problems that antihypertensive drugs cannot meet the clinical treatment requirements, achieve good application development prospects, and improve the effect of solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
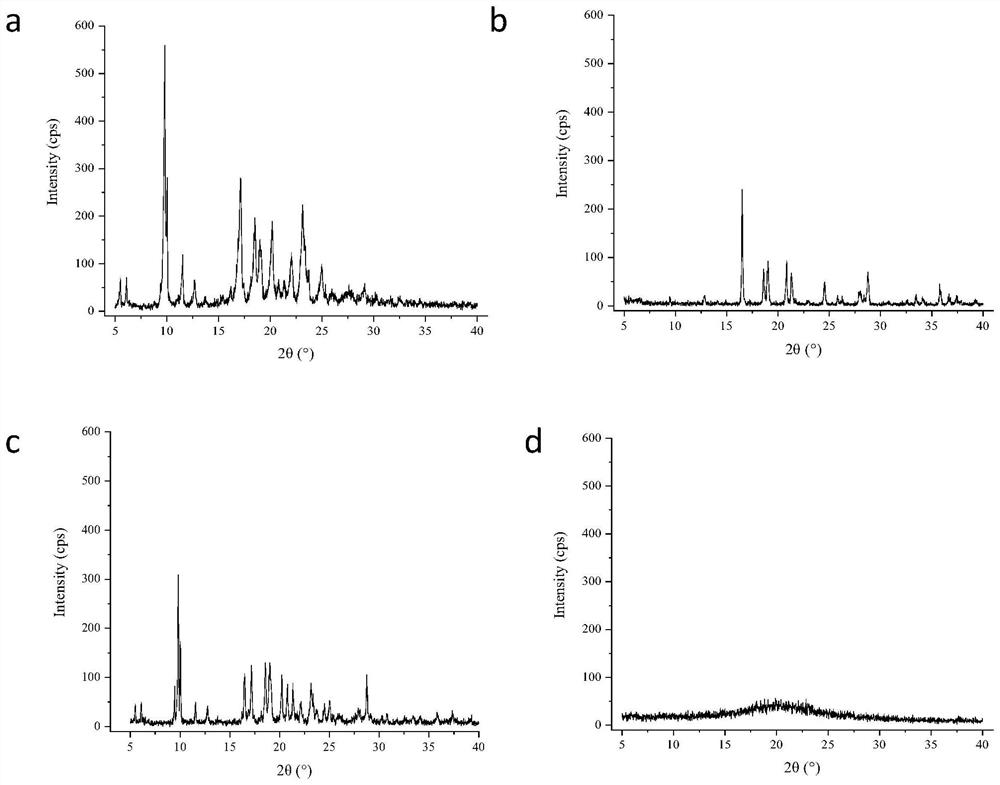
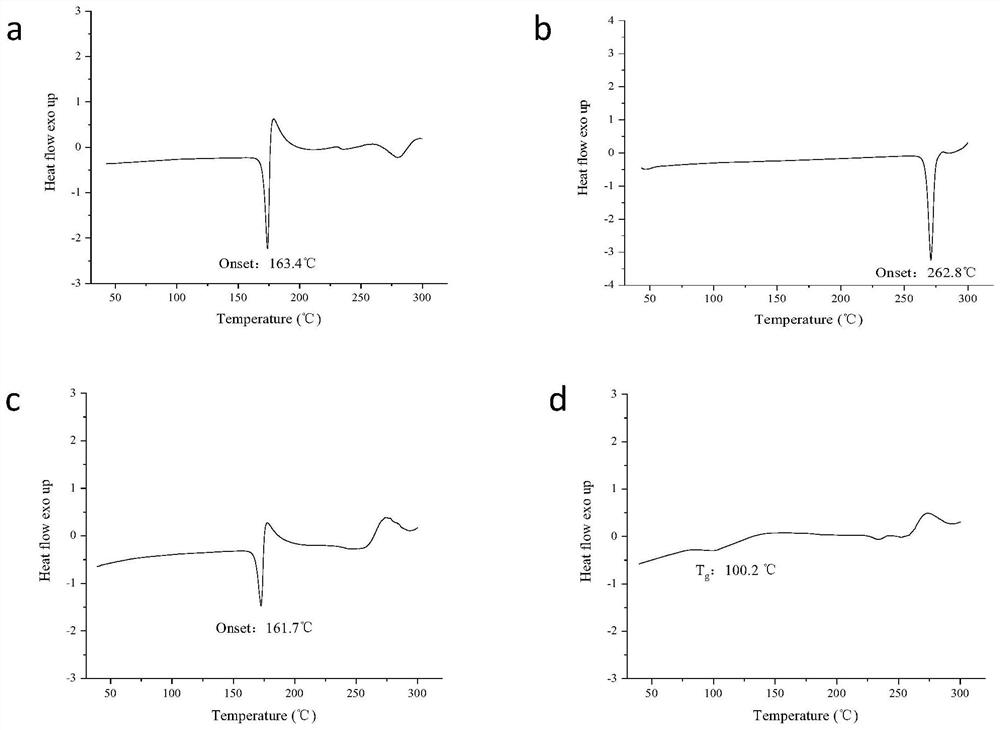
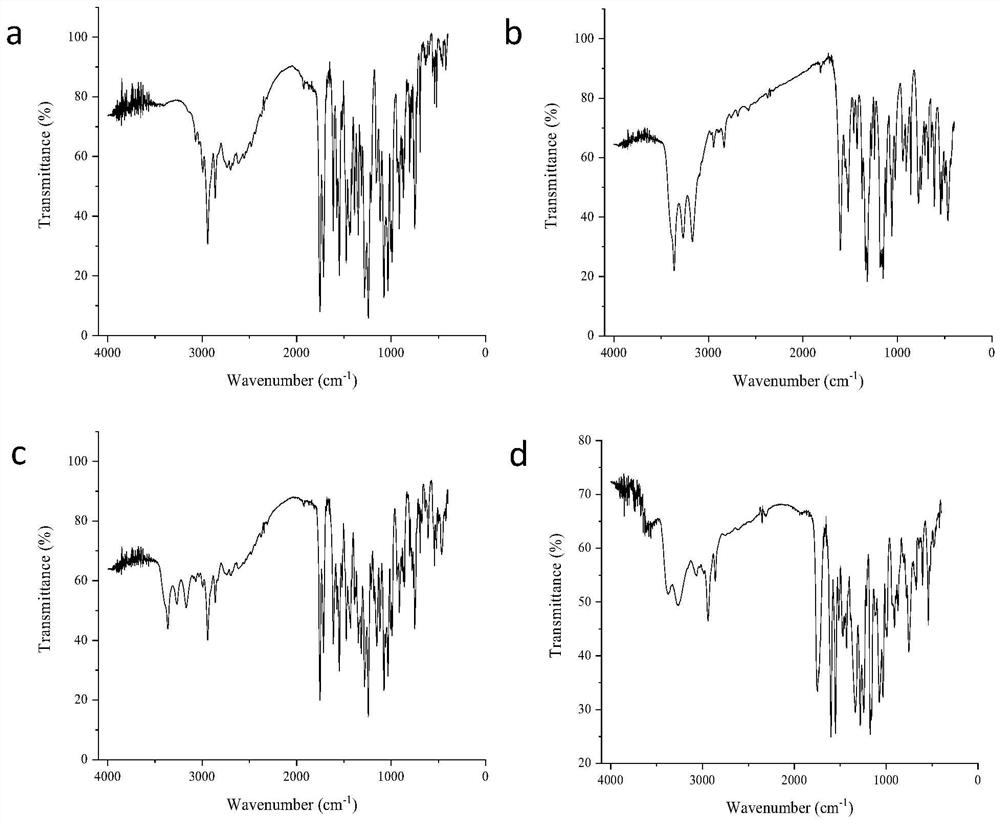
[0036] Preparation of co-amorphous substance of candesartan cilexetil and hydrochlorothiazide
[0037] Weigh about 0.305g of candesartan cilexetil crystals and 0.149g of hydrochlorothiazide crystals into the same 100mL eggplant-shaped bottle, add about 40mL of ethanol, and shake thoroughly to obtain a clear and transparent solution. The solution was placed on a rotary evaporator, the temperature of the water bath was set at 55 °C, and the ethanol was removed by rotary evaporation under reduced pressure to obtain the corresponding solid product. It was dried in a vacuum oven for 48 h to remove the residual solvent in the rotary evaporated product.
Embodiment 2
[0039] Preparation of co-amorphous substance of candesartan cilexetil and hydrochlorothiazide
[0040] Weigh about 1.221g of candesartan cilexetil crystals and 0.149g of hydrochlorothiazide crystals respectively into the same 100mL eggplant-shaped bottle, add about 50mL of methanol, and shake fully to obtain a clear and transparent solution. The solution was placed on a rotary evaporator, the temperature of the water bath was set at 35 °C, and the methanol was removed by rotary evaporation under reduced pressure to obtain the corresponding solid product. It was dried in a vacuum oven for 48 h to remove the residual solvent in the rotary evaporated product.
Embodiment 3
[0042] Preparation of co-amorphous substance of candesartan cilexetil and hydrochlorothiazide
[0043] Weigh about 0.305g of candesartan cilexetil crystals and 0.595g of hydrochlorothiazide crystals into the same 100mL eggplant-shaped bottle, add about 60mL of acetonitrile, and shake thoroughly to obtain a clear and transparent solution. The solution was placed on a rotary evaporator, the temperature of the water bath was set at 65 °C, and the acetonitrile was removed by rotary evaporation under reduced pressure to obtain the corresponding solid product. It was dried in a vacuum oven for 48 h to remove the residual solvent in the rotary evaporated product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com