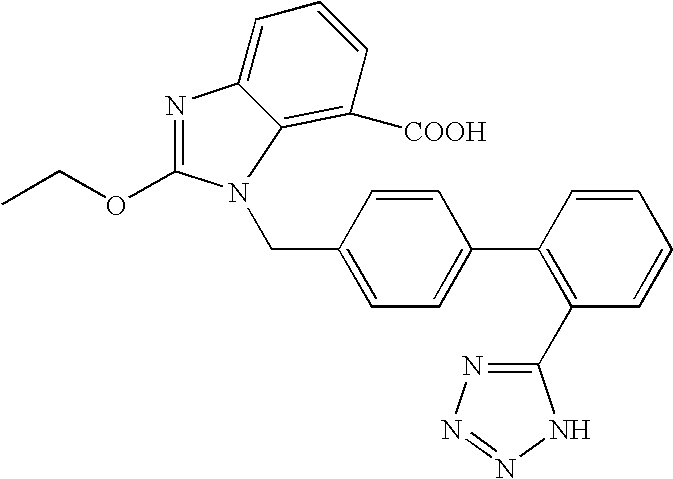
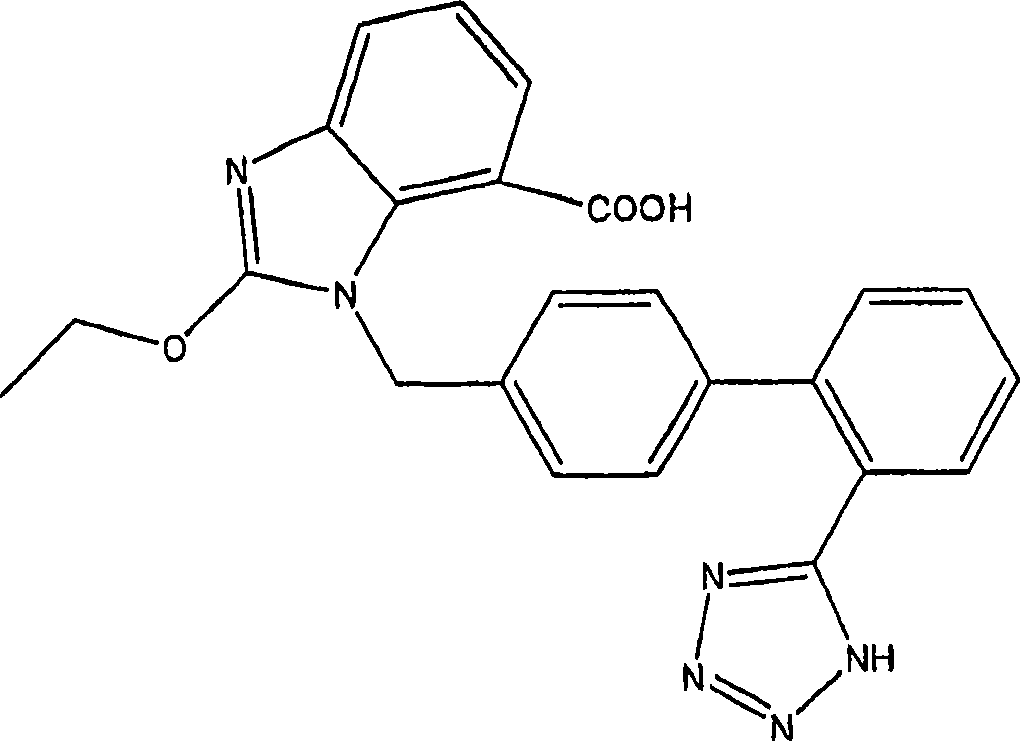
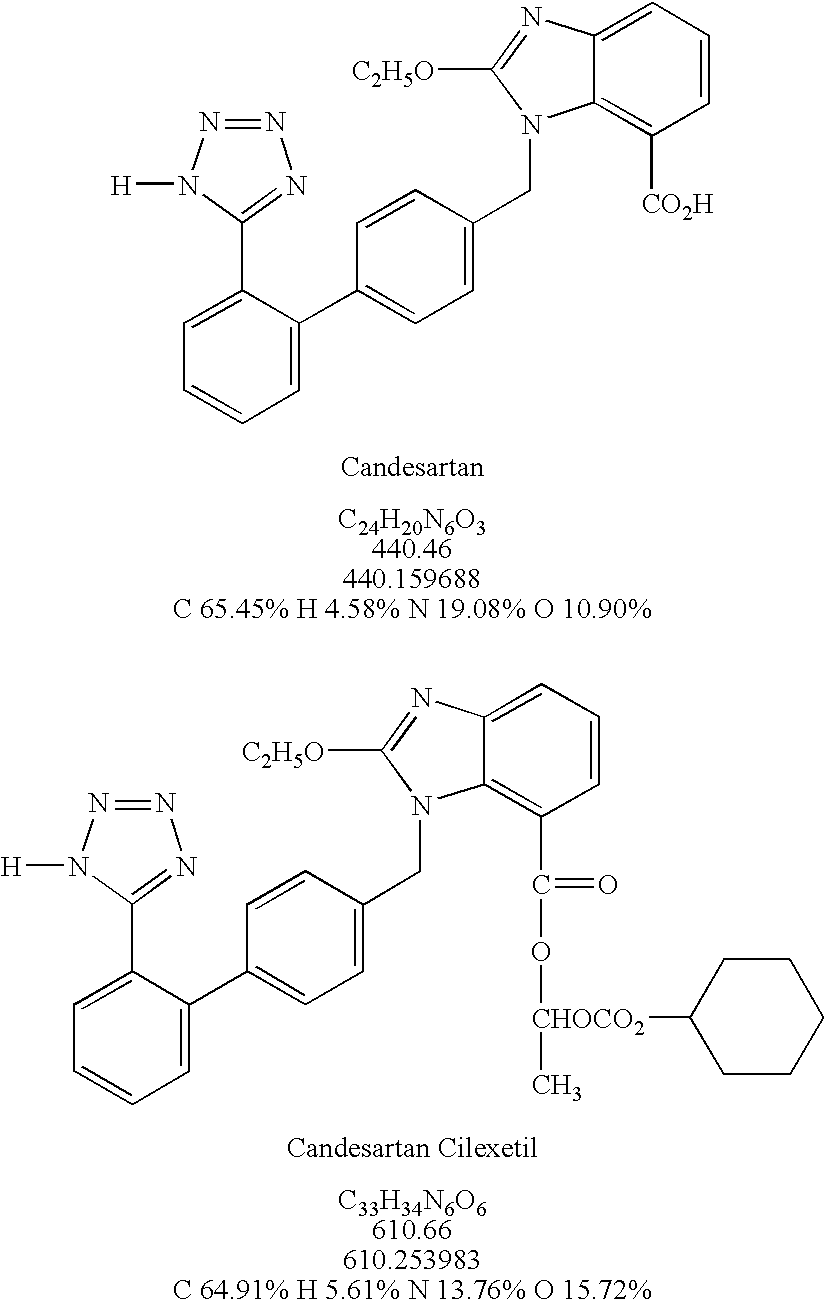
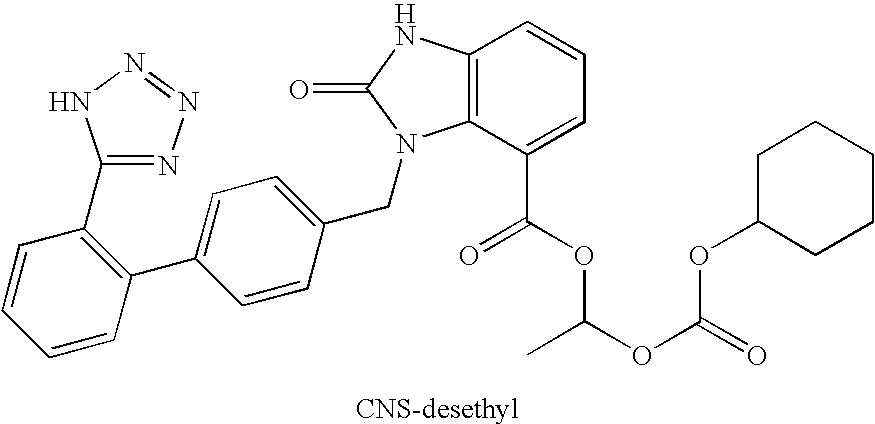
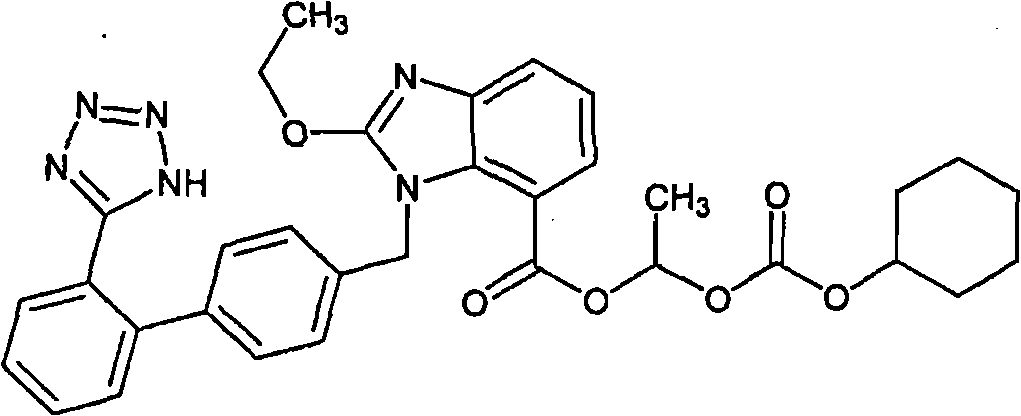
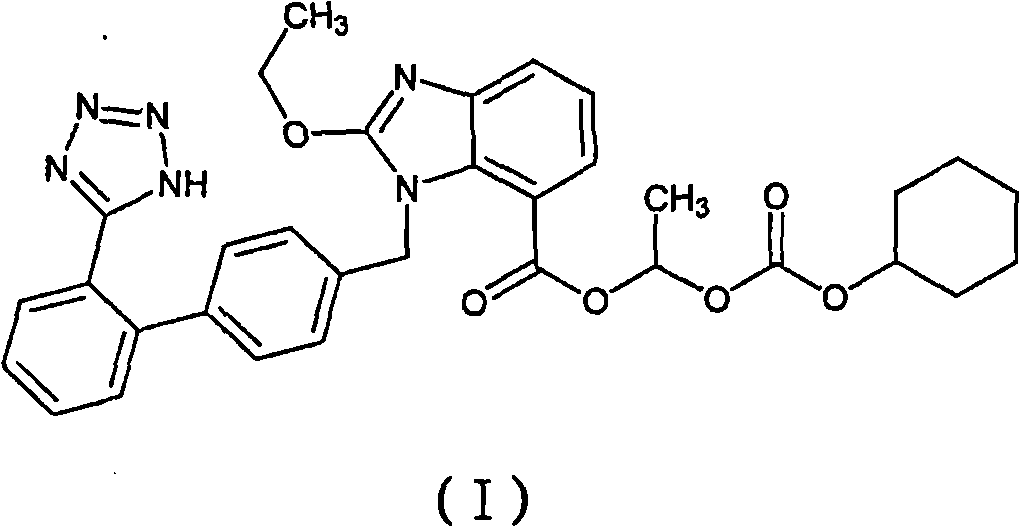
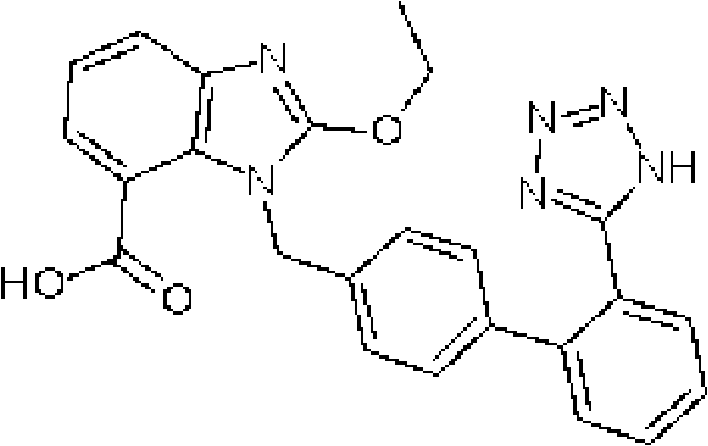
Patents
Literature
39 results about "Candesartan cilexitil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanoparticulate candesartan formulations
InactiveUS20060165806A1Improve pharmacokineticsUseful in treatmentPowder deliveryOrganic active ingredientsCandesartan cilexitilTreatment hypertension
The present invention is directed to compositions comprising a candesartan, such as candesartan cilexitil. The candesartan particles of the composition have an effective average particle size of less than about 2000 nm. The candesartan compositions of the invention are useful in the treatment of hypertension or related cardiovascular conditions.
Owner:ELAN PHRMA INT LTD
Nanoparticulate candesartan formulations
The present invention is directed to compositions comprising a candesartan, such as candesartan cilexitil. The candesartan particles of the composition have an effective average particle size of less than about 2000 nm. The candesartan compositions of the invention are useful in the treatment of hypertension or related cardiovascular conditions.
Owner:ELAN PHRMA INT LTD
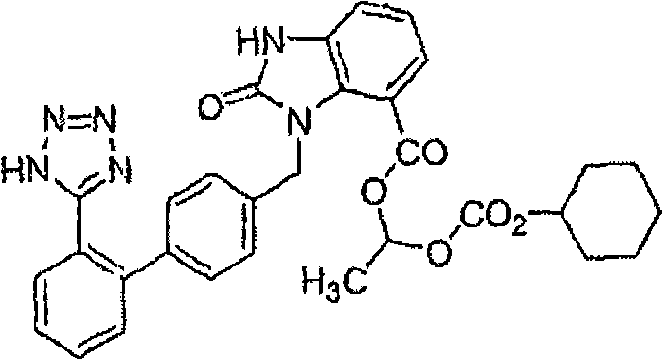
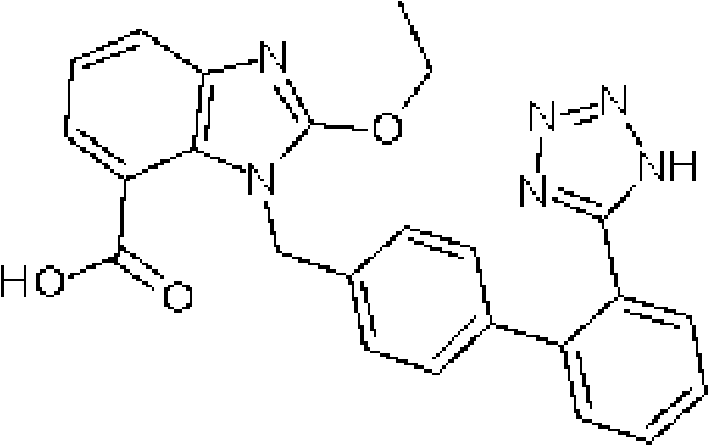
Preparation of candesartan cilexetil in high purity
The present invention is directed to the preparation of substantially pure candesartan cilexetil by the deprotection of trityl candesartan cilexetil and crystallization and / or recrystallization of candesartan cilexetil.
Owner:TEVA PHARM USA INC
Stable micronized candesartan cilexetil and methods for preparing thereof
The invention encompasses sable candesartan cilexetil of fine particle size, wherein desethyl-candesartan (desethyl-CNS) within the stable candesartan cilexetil does not increase to more than about 0.1% w / w by HPLC relative to the initial amount of candesartan cilexetil, when the stable candesartan cilexetil is maintained at a temperature of about 55° C. for at least 2 weeks, methods of making the same and pharmaceutical compositions thereof.
Owner:TEVA PHARM USA INC
Stable candesartan cilexetil amlodipine pharmaceutical composition and its preparation method
ActiveCN102743381AShorten production timeSave labor hoursOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanPolyethylene glycol
The invention relates to a stable candesartan cilexetil and amlodipine pharmaceutical-containing composition and its preparation method. The invention is characterized in that a) each tablet contains 4mg-8mg of candesartan cilexetil, b) each tablet contains 2-10mg of amlodipine or its pharmaceutically acceptable salt, c) each tablet contains polyethylene glycol, wherein the weight of polyethylene glycol accounts for 2-10% of the tablet, and d) the tablet is prepared by a compressed tablet technology by using a dry method.
Owner:福安药业集团庆余堂制药有限公司
Detection method of nitrosamine impurities in candesartan cilexetil
InactiveCN112129853AEnsure quality and safetyEnsure medication safetyComponent separationFluid phaseChemical compound
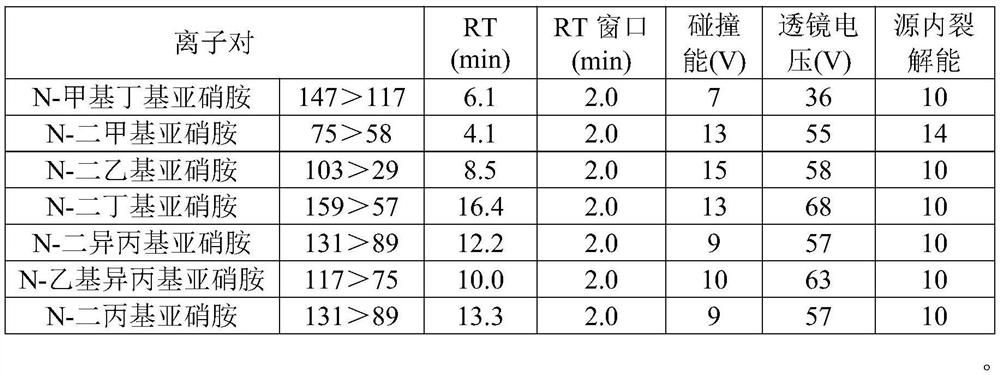
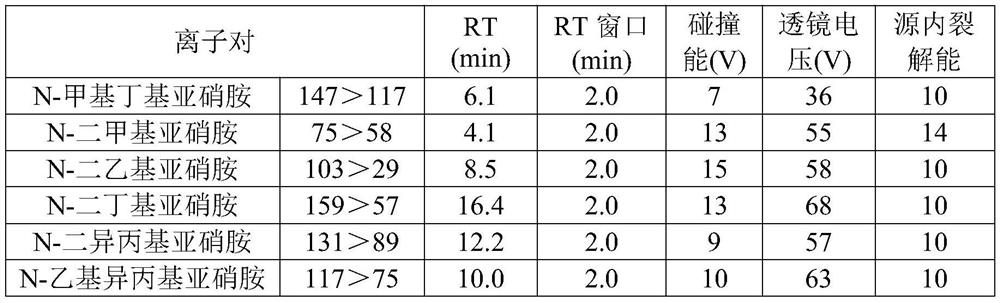
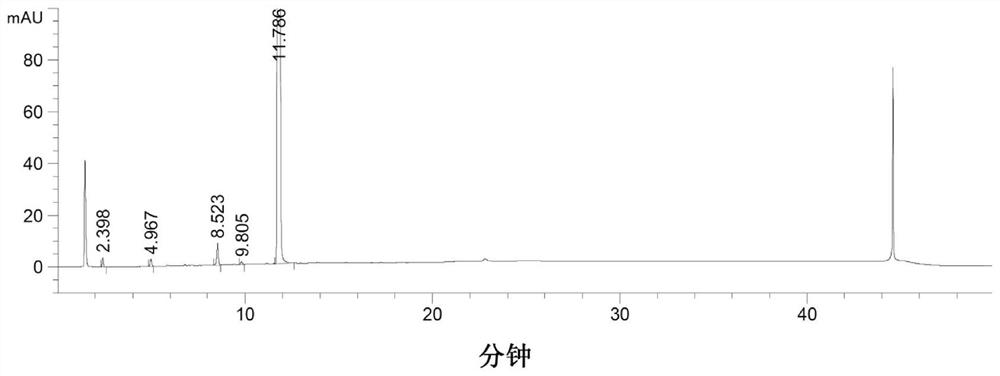
The invention discloses a detection method of nitrosamine impurities in candesartan cilexetil. The method comprises the following steps of: S1, preparing a solution: respectively preparing a test solution and a standard curve solution; and S2, detecting: detecting and analyzing the test solution and the standard curve solution in the step S1 by adopting liquid chromatography and mass spectrometry.According to the detection method disclosed by the invention, nitrosamine compounds in the candesartan cilexetil can be more comprehensively detected, and simultaneous detection of seven nitrosaminecompounds can be realized on the premise of adopting one ion source, so that the quality and medication safety of the candesartan cilexetil are ensured. The detection limit of the detection method fordetecting the nitrosamine compounds in the candesartan cilexetil can reach a trace level; the detection method disclosed by the invention has good system adaptability, specificity, quantification limit and detection limit, linearity and range, accuracy, precision, durability and solution stability on detection of seven nitrosamines in the candesartan cilexetil.
Owner:HINYE PHARM CO LTD
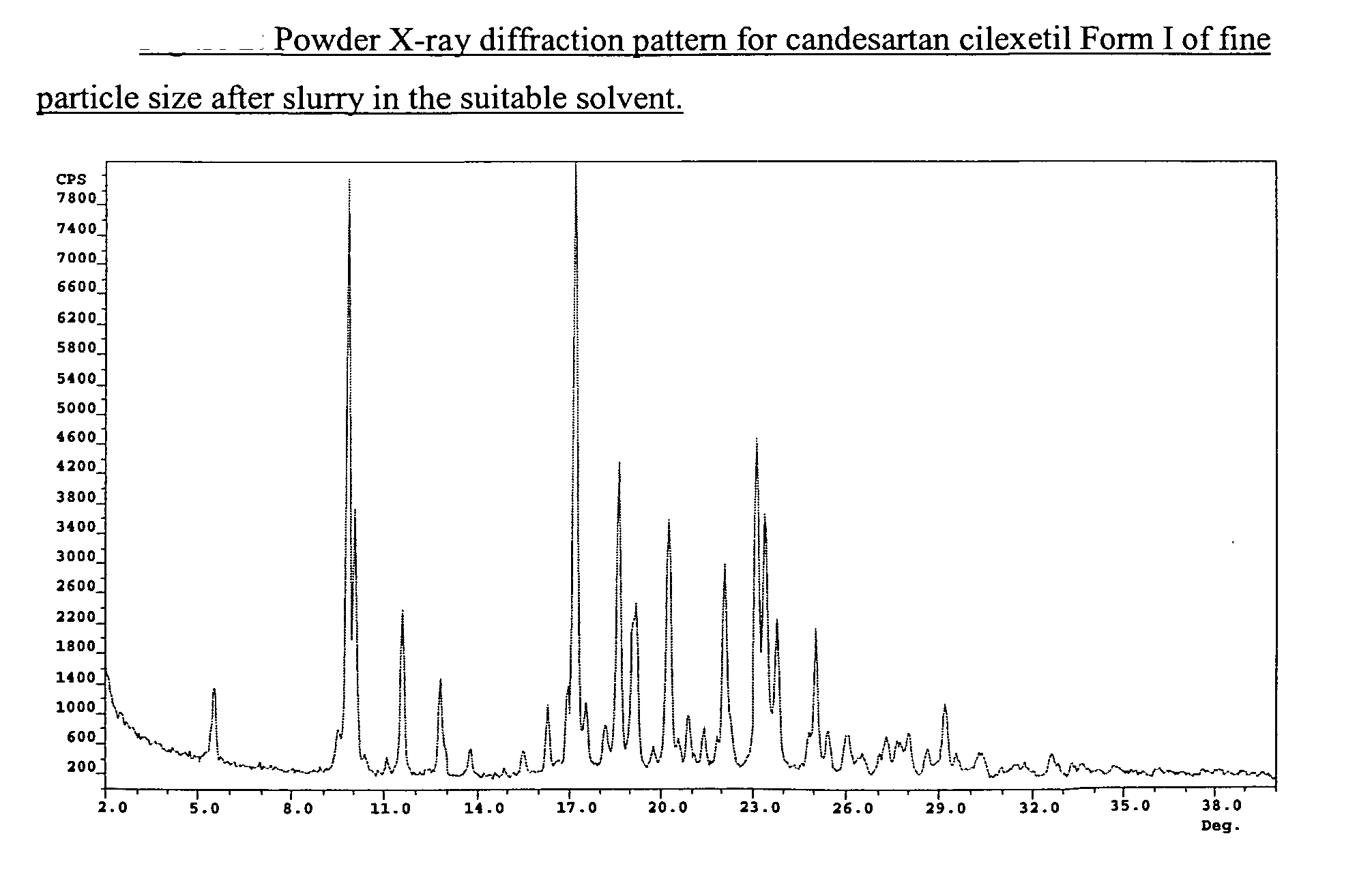
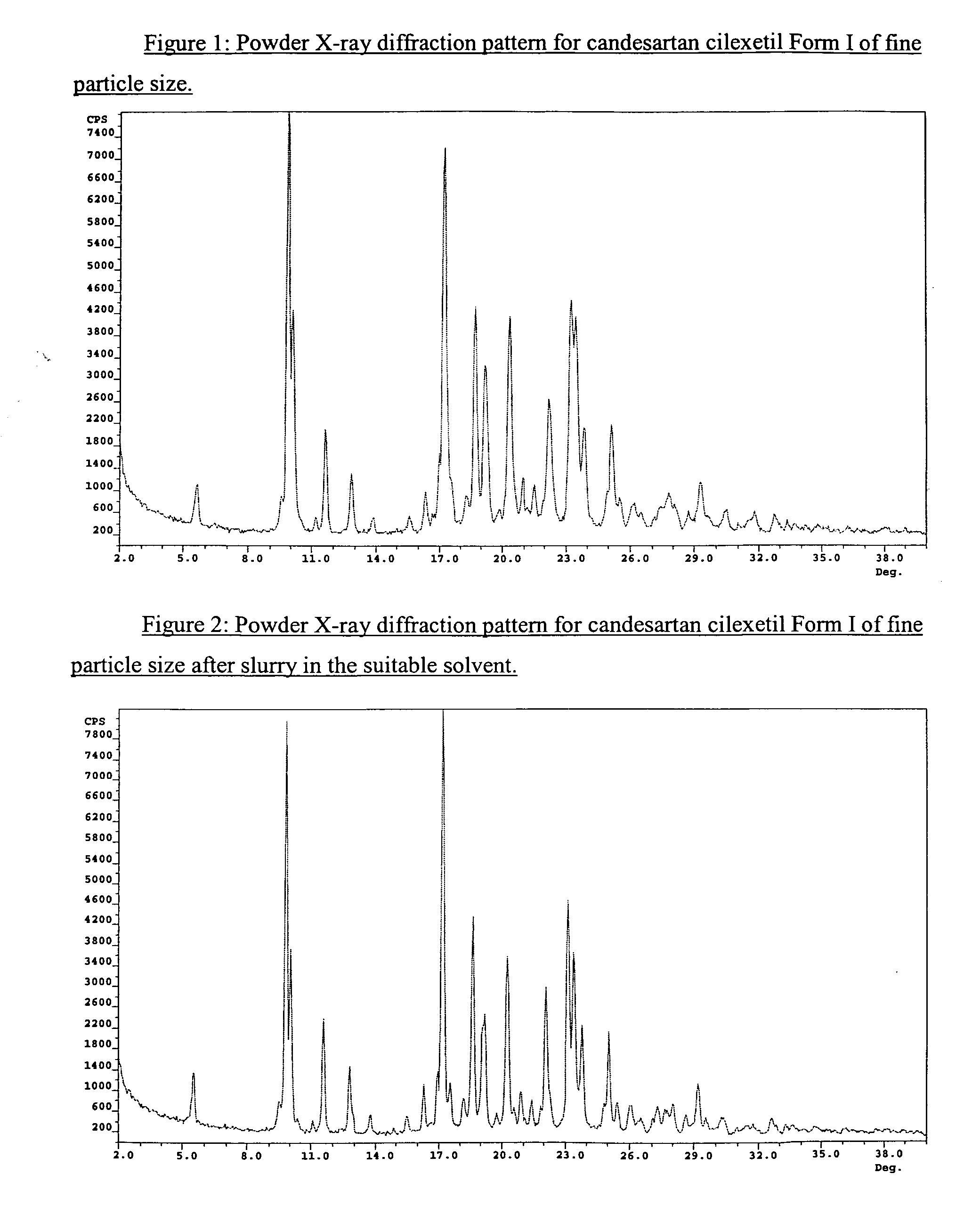
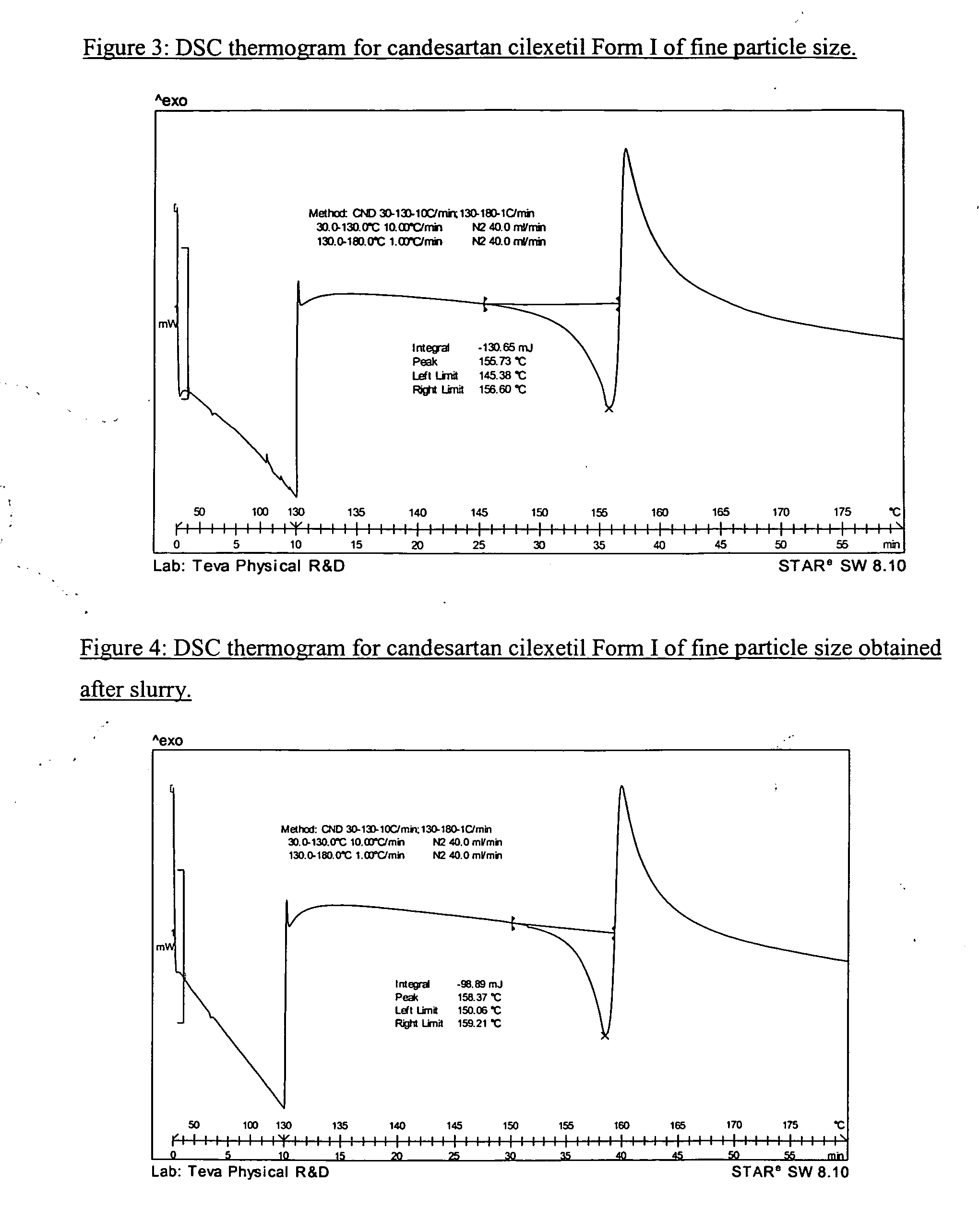
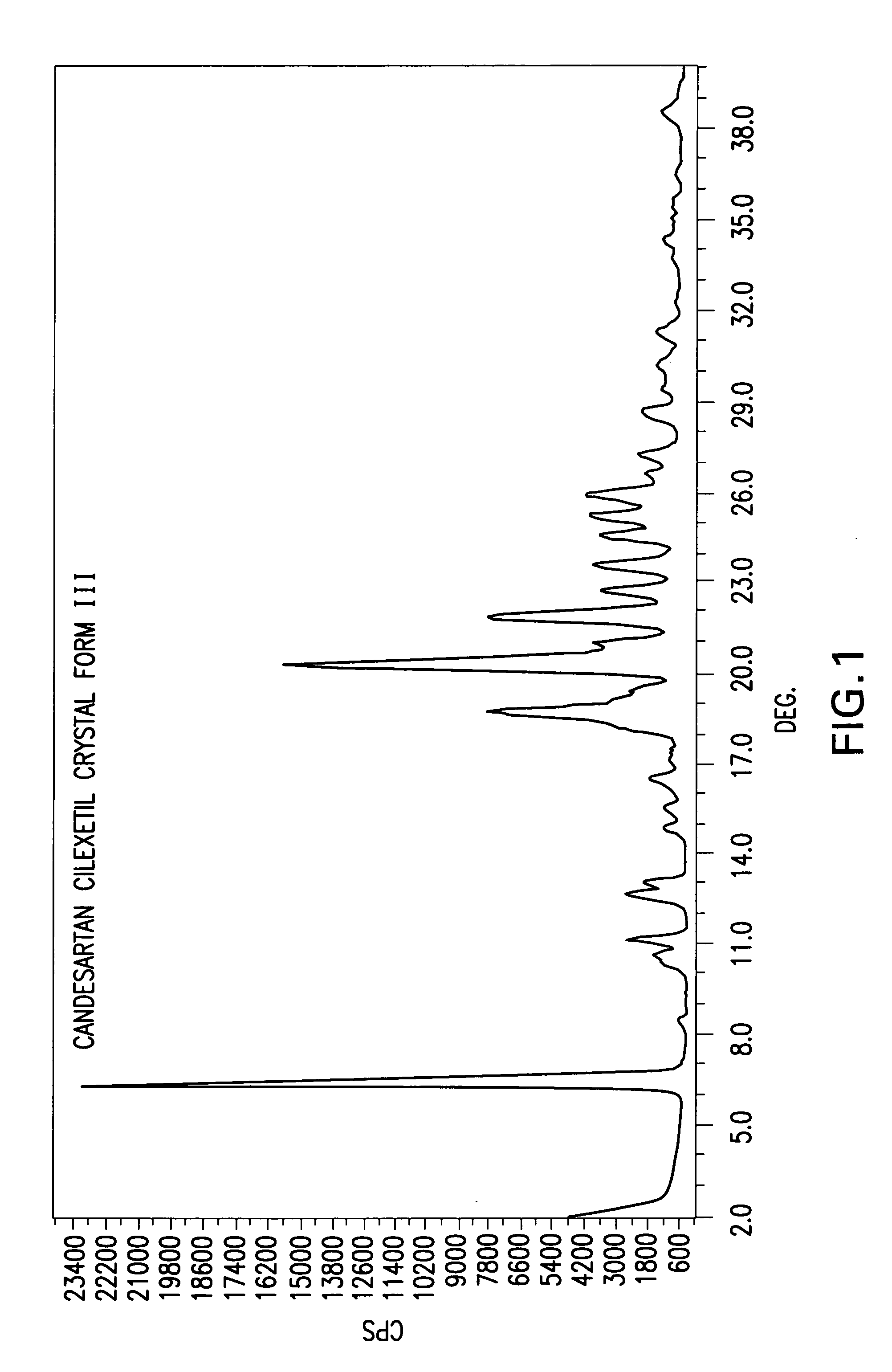
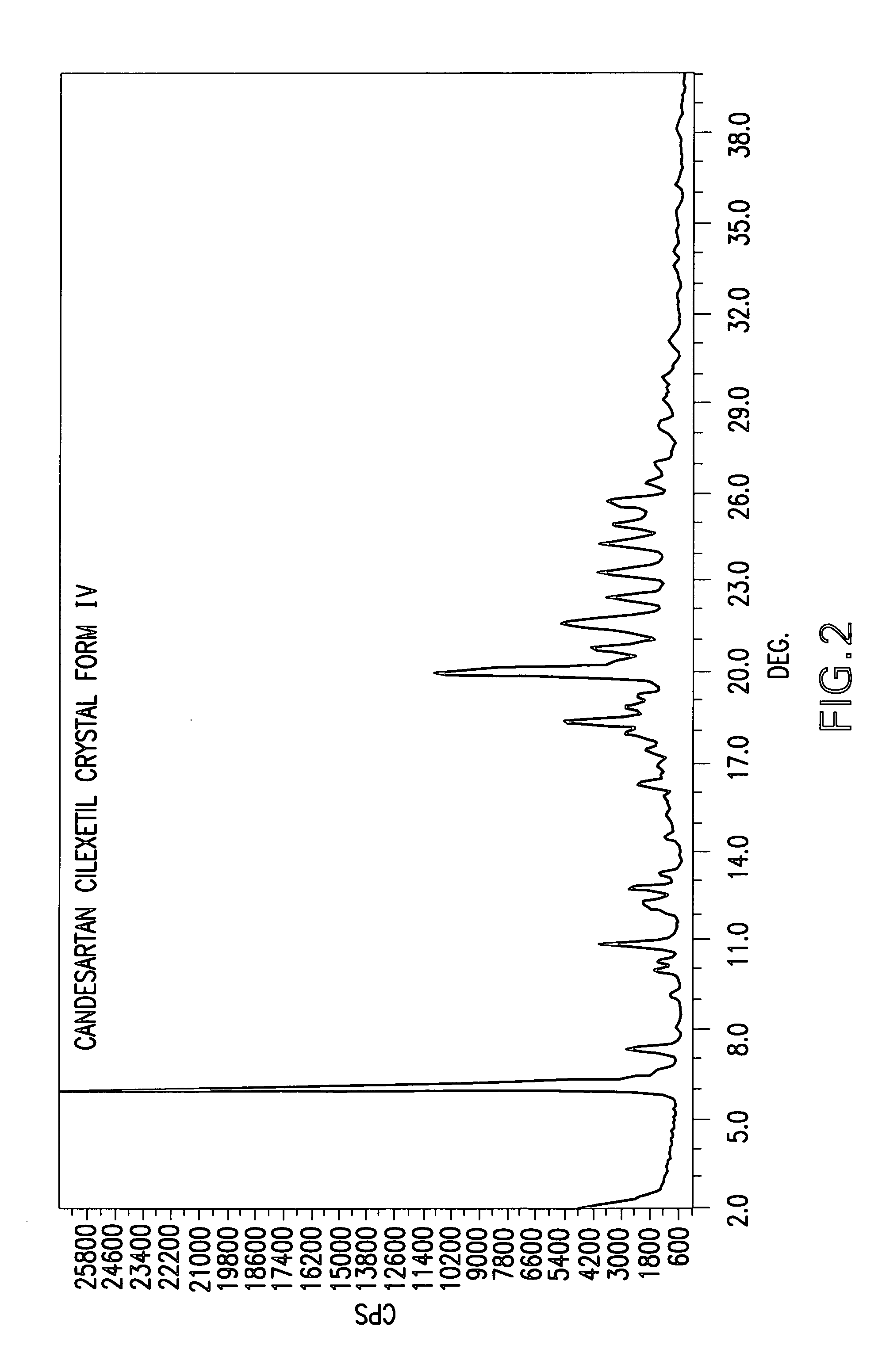
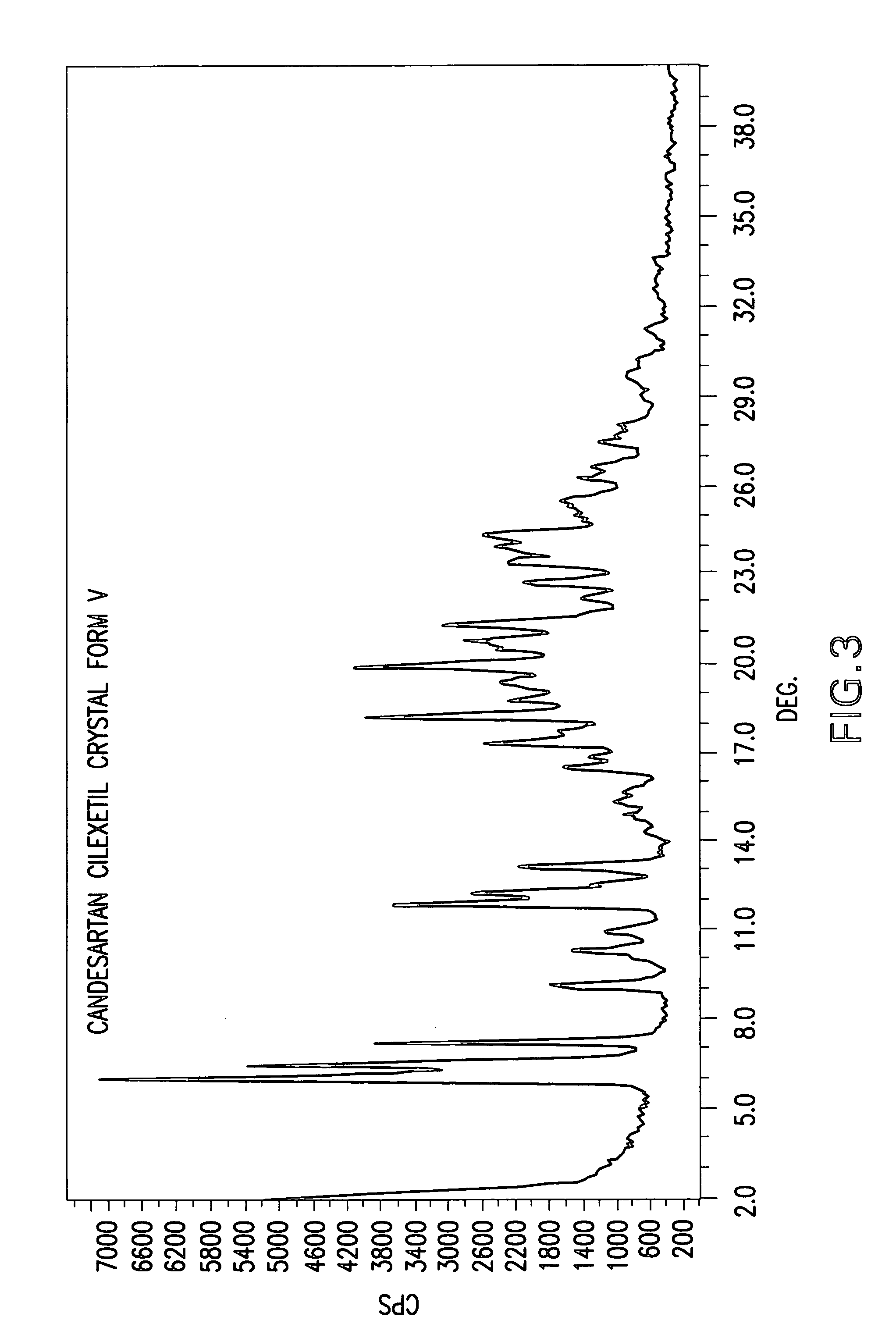
Candesartan cilexetil polymorphs
Provided are candesartan cilexetil forms and methods of their preparation. Also provided are pharmaceutical compositions prepared by combining at least one pharmaceutically-acceptable excipient with at least one candesartan cilexetil form of the invention.
Owner:TEVA PHARM USA INC
Process for the Preparation of Candesartan Cilexetil
InactiveUS20100197933A1High yieldShort reaction timeOrganic chemistryBulk chemical productionDiseaseOrganic solvent
The present invention provides an improved synthesis for the manufacture of candesartan and pharmaceutically acceptable salts and esters thereof as active ingredients of a medicament for the treatment of hypertension and related diseases and conditions which comprises the removal of the tetrazolyl protecting group in an organic solvent, and in the presence of a Lewis acid.
Owner:ZUPANCIC SILVO
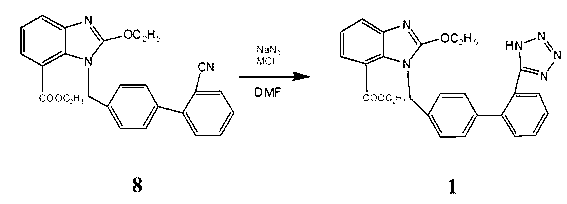
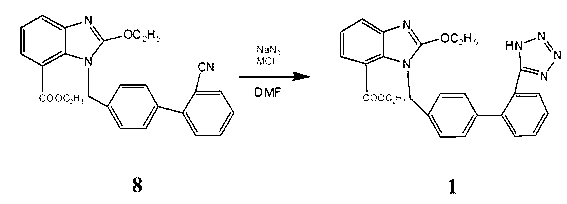
Synthesis process of candesartan cilexetil
The invention relates to a process improving method of candesartan cilexetil; 1-[(2'-cyano-1, 1'-xenyl-4-) methyl]-2-ethyoxyl-7-benzimidazole carboxylic acid ethyl ester and sodium azide serve as raw materials, N,N-dimethyl formamide serves as solvent, MC1 serves as catalyst, and the materials are synthesized into candesartan cilexetil at the temperature of 130DEG C to 160DEG C, wherein M is alkali metal or an ammonium cation. According to the method, the price of the used catalyst is low, no toxin or little toxin is produced, the production cost and the environmental treatment cost are reduced, the treatment after reaction is simple, the yield is higher, and the industrialized production is facilitated.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of candesartan cilexetil crystal form I spherical crystal
ActiveCN106397409AConducive to stable productionHigh crystallinityOrganic chemistry methodsAlcoholOrganic solvent
The invention relates to a preparation method of a candesartan cilexetil crystal form I spherical crystal, and belongs to the technical field of crystallization. The technical scheme adopted by the invention is that the preparation method comprises the following steps: firstly, adding candesartan cilexetil into ketone solvents, wherein the solid-liquid ratio of a solution is 0.01 to 0.1g / ml; stirring and dissolving at the temperature of 20 to 30DEG C, and continuously stirring for 30 to 60 minutes; secondly, filtering and decoloring; transferring filtrate into a crystallizer, controlling the temperature of a system to be 10 to 20DEG C, adding alcohol organic solvents, performing dilution crystallization, filtering, washing and drying to obtain a finished product disclosed by the invention. The candesartan cilexetil crystal form I spherical crystal by the preparation method provided by the invention is good in flowability and facilitates stable production of a preparation process.
Owner:迪嘉药业集团股份有限公司
Oral candesartan cilexetil solid preparation and preparation method thereof
InactiveCN103301121AImprove stabilityImprove bioavailabilityOrganic active ingredientsPill deliveryCyclodextrinPharmaceutical medicine
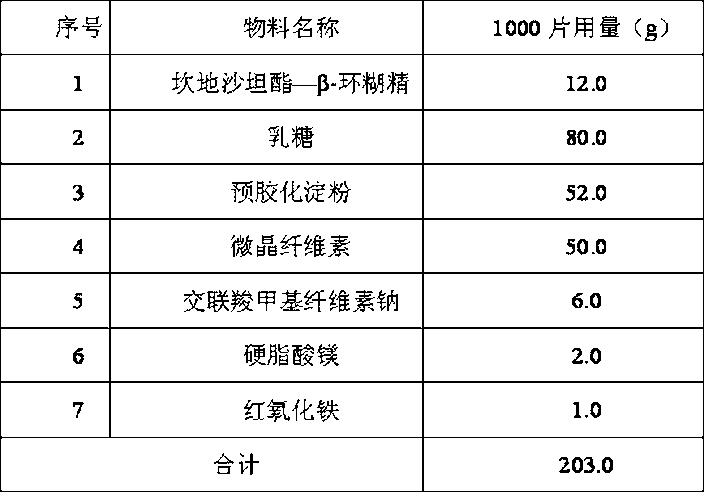
The invention discloses an oral candesartan cilexetil solid preparation and a preparation method thereof. The oral solid preparation is prepared from candesartan cilexetil, stabilizer and pharmaceutically acceptable auxiliary ingredients, wherein the stabilizer is beta-cyclodextrin, and the weight of the stabilizer is 0.3 to 3 times that of the candesartan cilexetil. The preparation method comprises the steps of mixing candesartan cilexetil, beta-cyclodextrin or derivatives thereof and red iron oxide and sieving twice with a 100-mesh sieve, and then adding proper pharmaceutical auxiliary ingredients to obtain the candesartan cilexetil preparation. According to the oral candesartan cilexetil solid preparation and the preparation method thereof, the candesartan cilexetil can be inhibited from decomposing and keep long-time stabilizing and the expiry date of the candesartan cilexetil preparation can be prolonged.
Owner:邓俐丽
Application of candesartan cilexetil or pharmaceutically acceptable salt thereof in preparation of drug for preventing and/or treating COVID-19
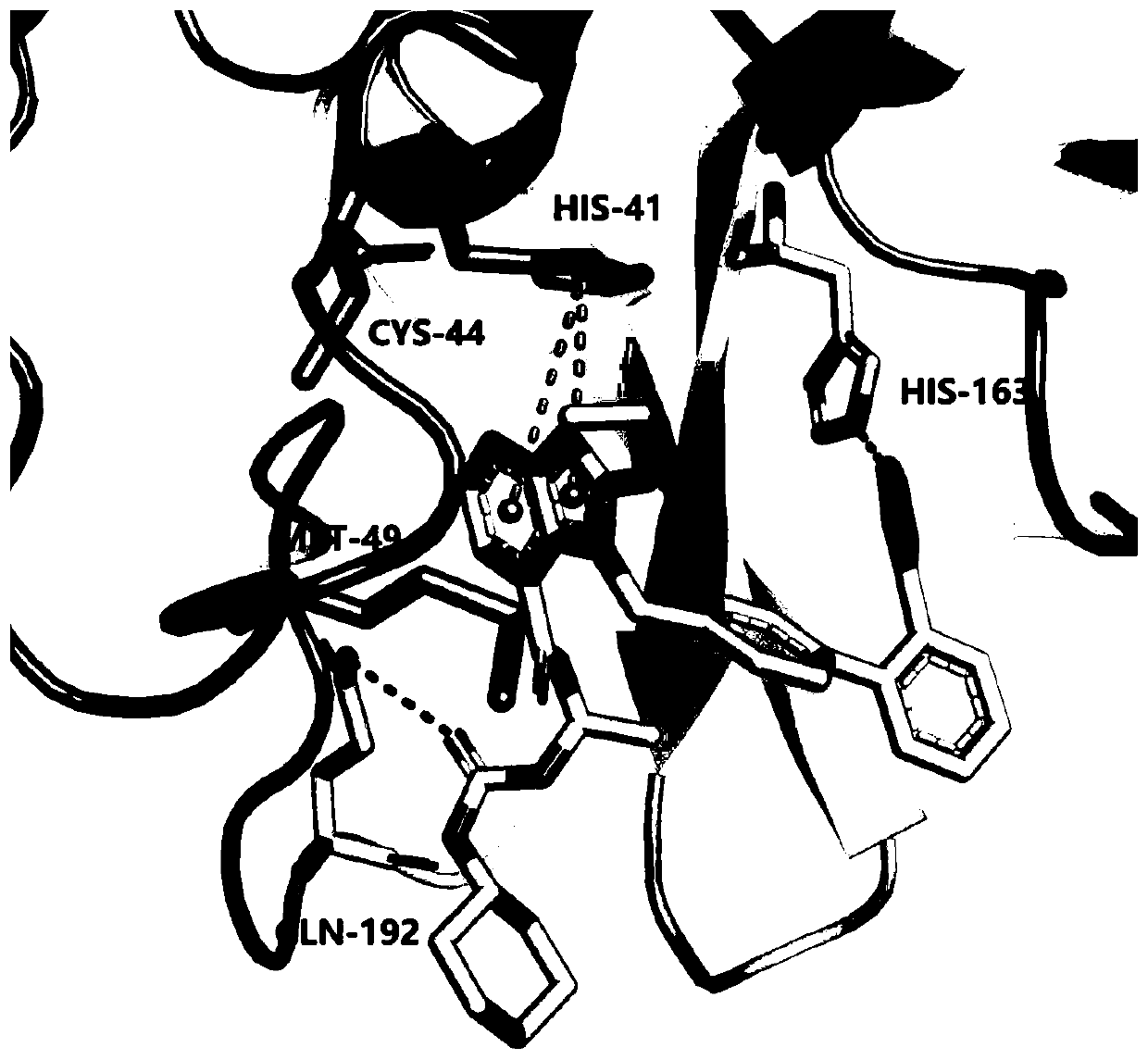
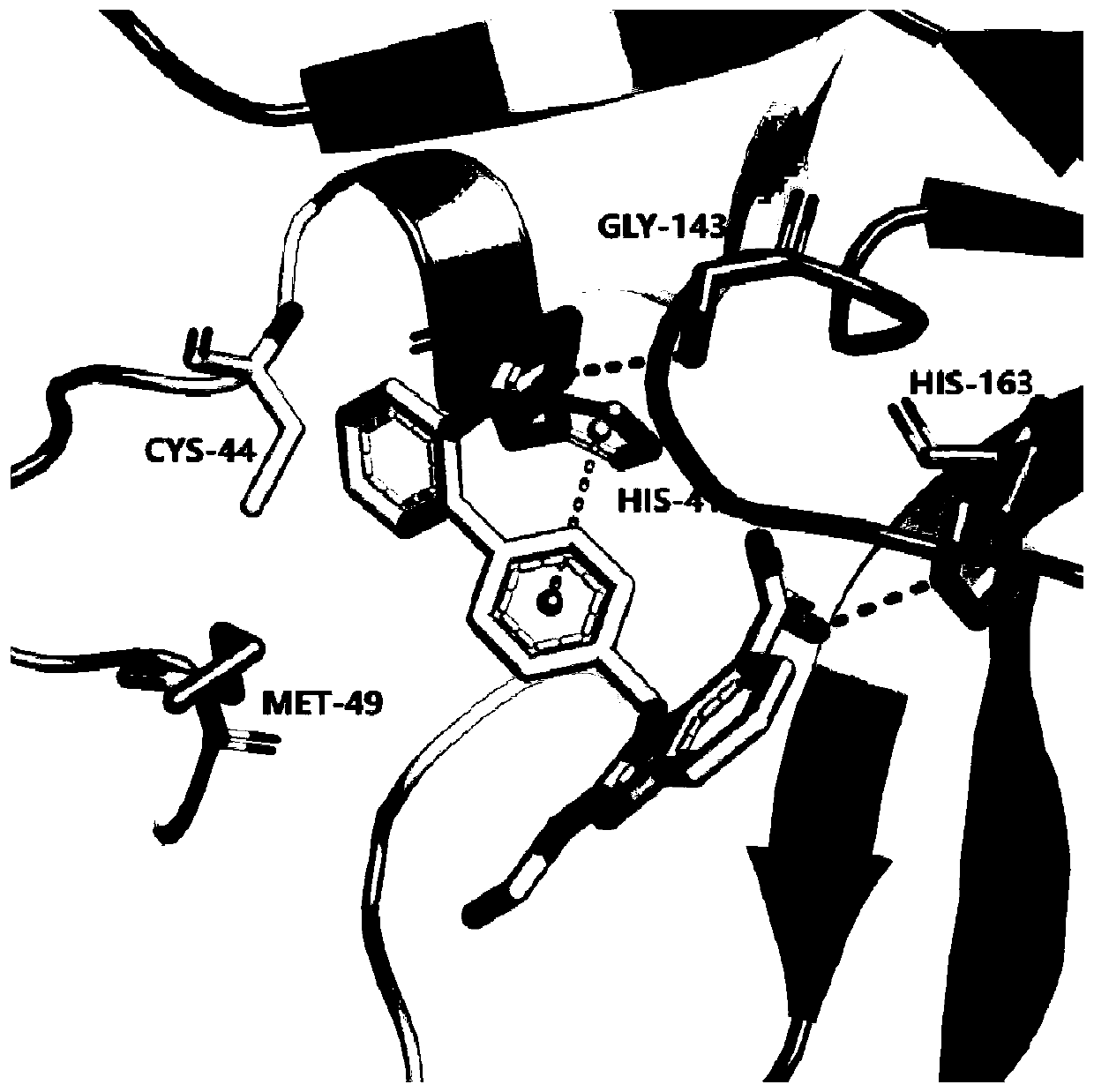
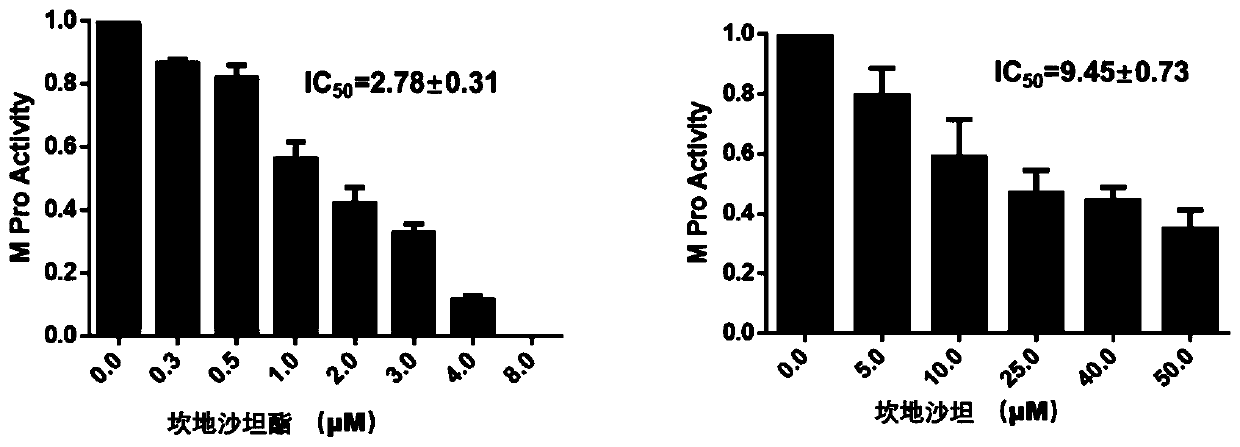
ActiveCN111419840AStrong binding strengthInhibitory activityOrganic active ingredientsAntiviralsHydrolysateMedicine
The invention discloses an application of candesartan cilexetil or a pharmaceutically acceptable salt thereof in preparation of a drug for preventing and / or treating COVID-19. The candesartan cilexetil and hydrolysate candesartan have stronger binding strength with SARS-CoV-2 target 3CL hydrolase (MPro) that causes inflammation in the lung, and can significantly inhibit the activity of the 3CL hydrolase, wherein the IC50 of the candesartan cilexetil and the hydrolysate candesartan against the 3CL hydrolase (Mpro) is 2.78+ / -0.31 [mu]M and 9.45+ / -0.73 [mu]M, respectively, and the results indicate that the candesartan cilexetil and the hydrolysate candesartan have the effect of preventing and treating pneumonia caused by the SARS-CoV-2, and can be used to prepare an anti-pneumonia drug for application.
Owner:SUN YAT SEN UNIV +1
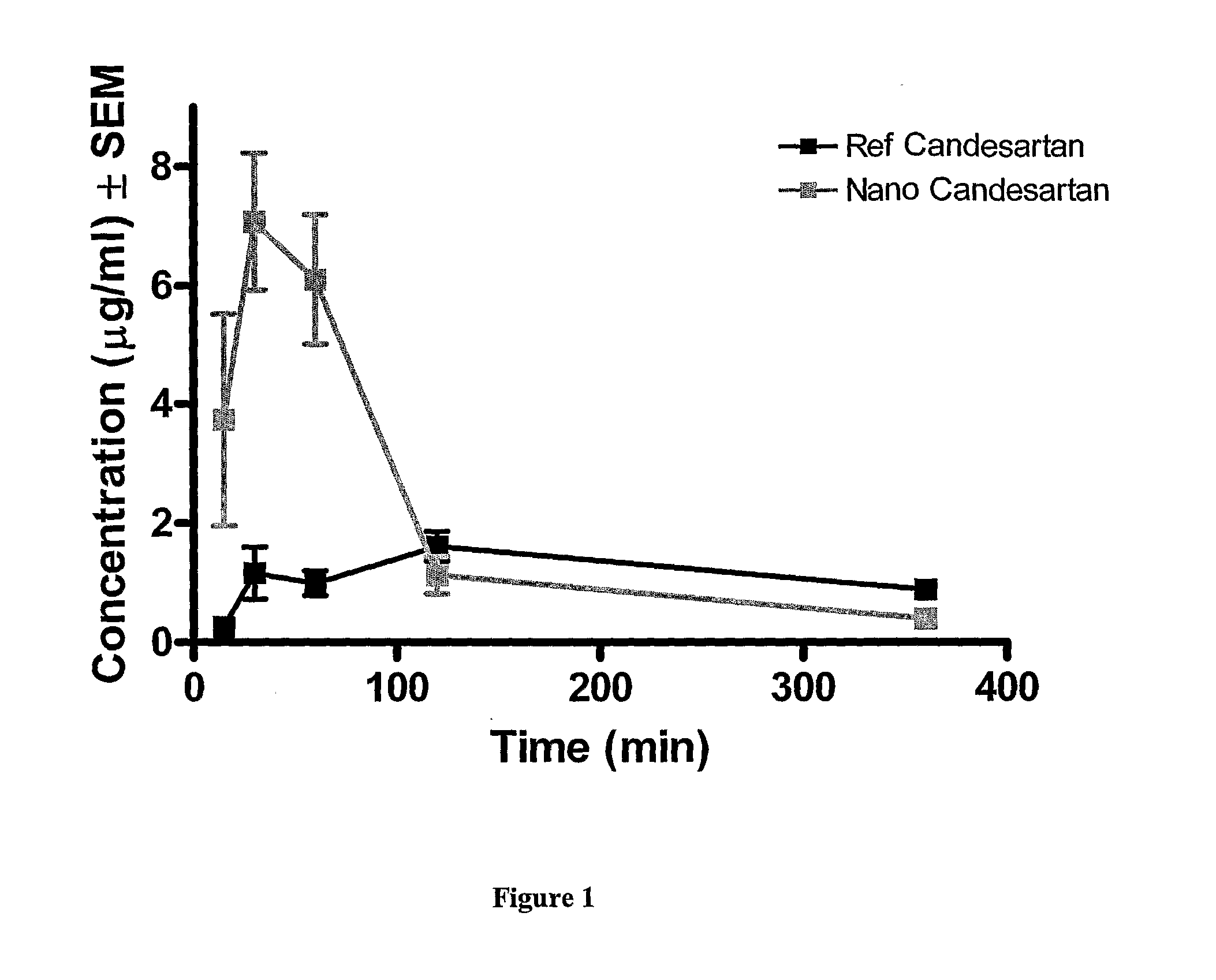
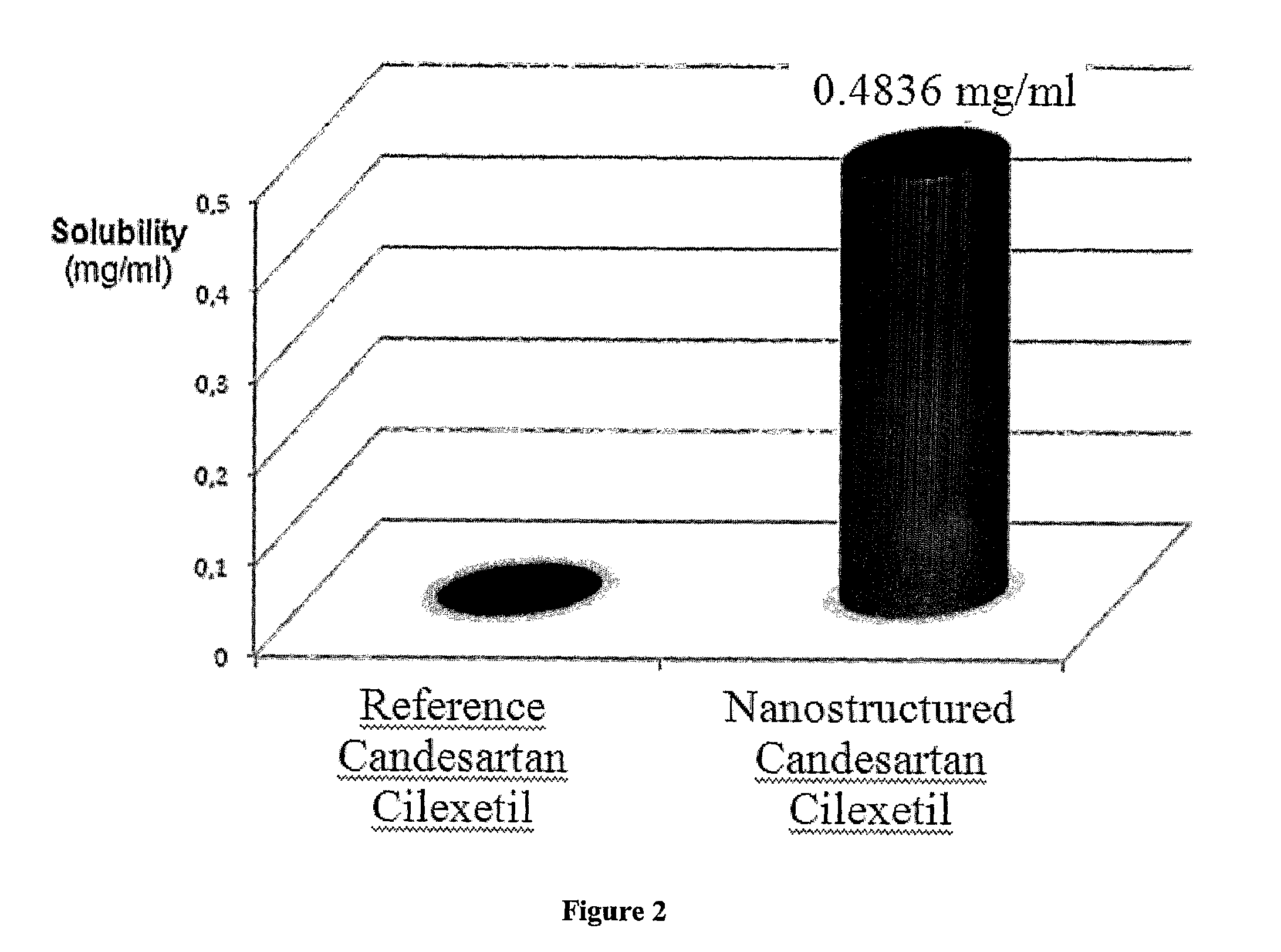
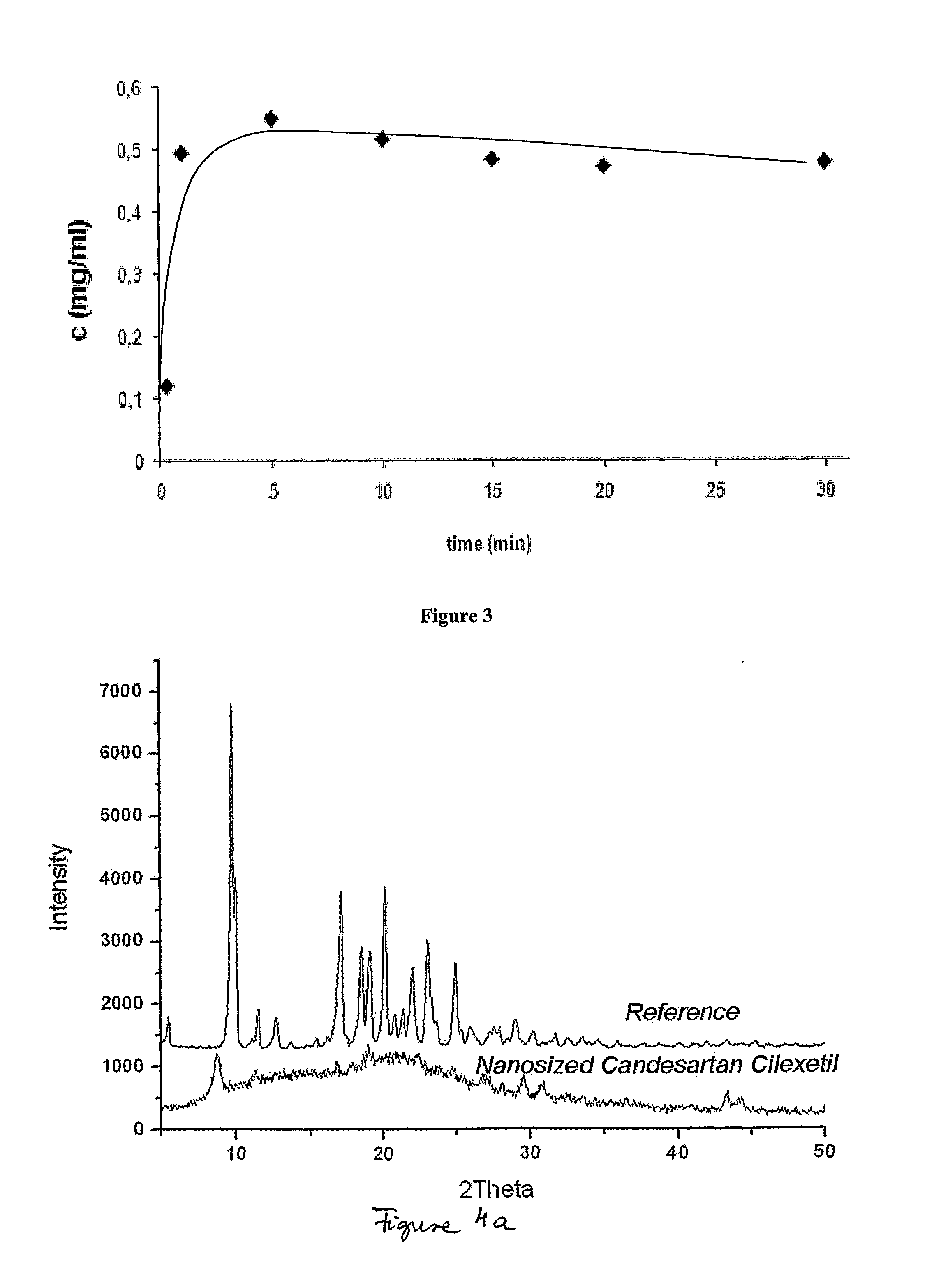
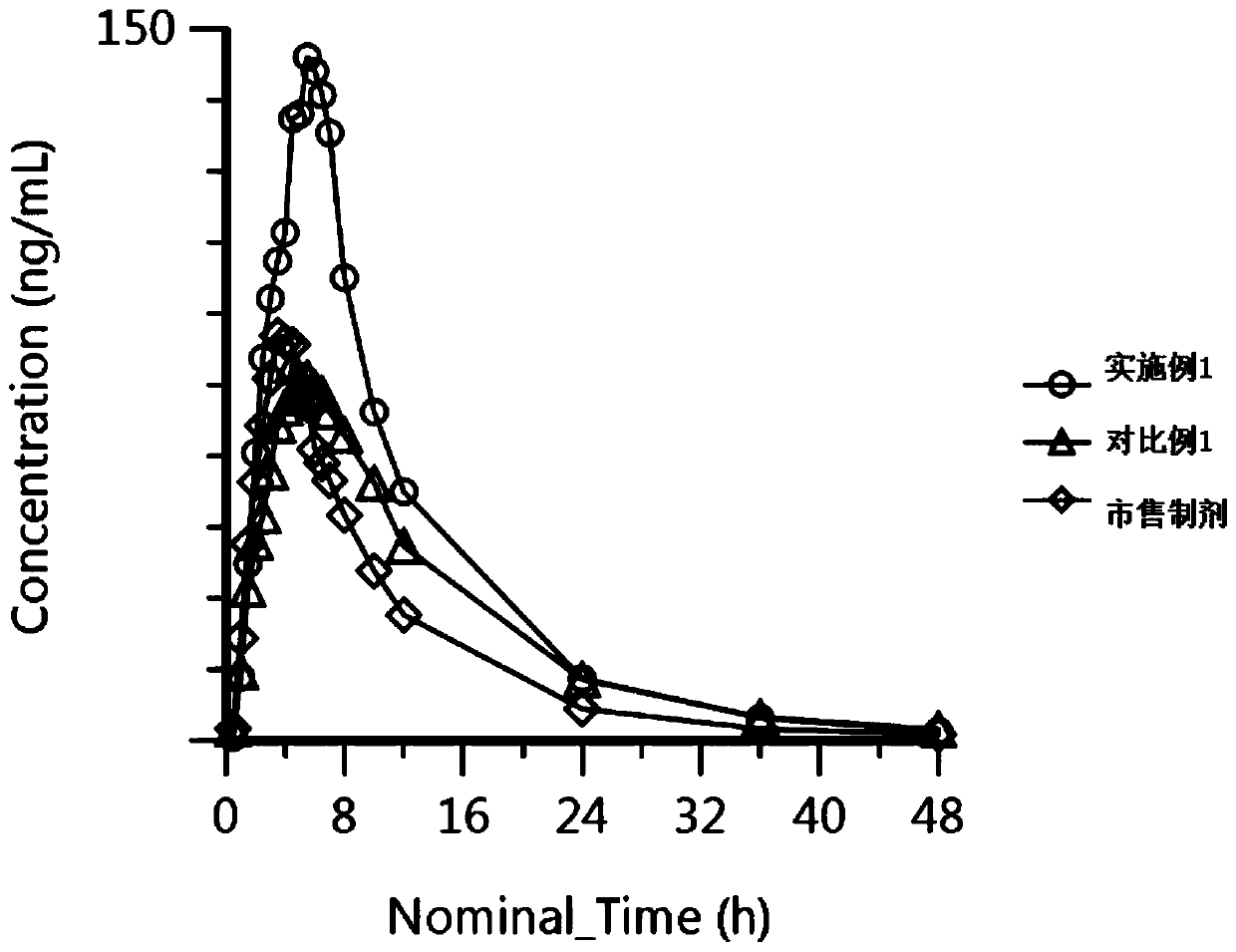
Nanoparticulate candesartan cilexitil compositions, process for the preparation thereof and pharmaceutical compositions containing them
InactiveUS20120141561A1Reduced bioavailabilityReduce the impactPowder deliveryBiocideNanoparticlePharmaceutical drug
The present invention is directed to nanostructured (nanoparticulated) Candesartan or its pharmaceutically acceptable ester, preferable Candesartan Cilexetil, or co-crystal compositions, process for the preparation thereof and pharmaceutical compositions containing them. The nanoparticles of Candesartan or its pharmaceutically acceptable ester, preferable Candesartan Cilexetil, or co-crystal according to the invention have an average particle size of less than about 500 nm. Candesartan Cilexetil is a prodrug, is hydrolyzed to Candesartan during absorption from the gastrointestinal tract. Candesartan is a selective AT1 subtype angiotensin II receptor antagonist.
Owner:DRUGGABILITY TECH IP HOLDCO JERSEY
Capsule preparation containing candesartan cilexetil and preparation method of capsule preparation
InactiveCN110917170AImprove bioavailabilityGood dissolution effectOrganic active ingredientsPharmaceutical non-active ingredientsBioavailabilityOrganic chemistry
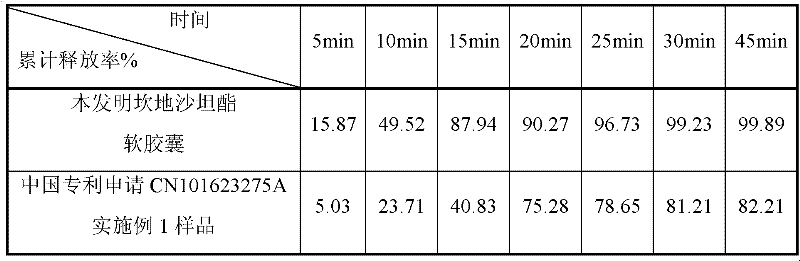
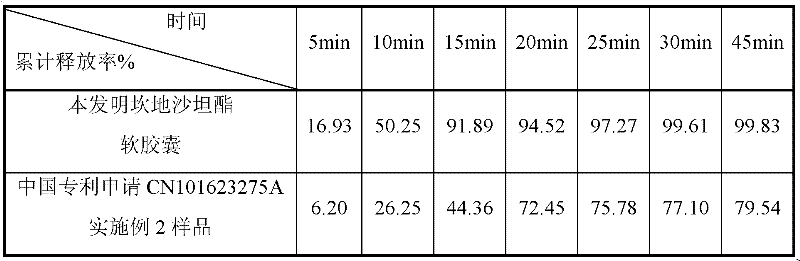
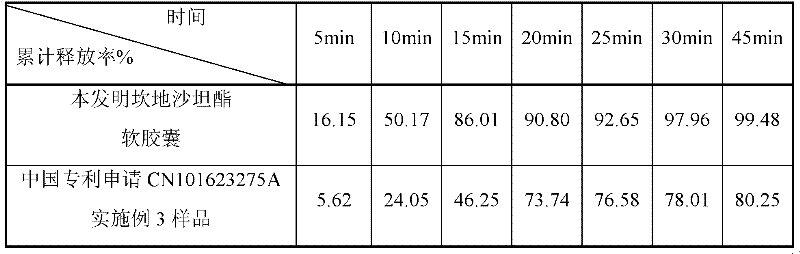
The invention belongs to the technical field of medicines and in particular discloses a capsule preparation containing candesartan cilexetil and a preparation method of the capsule preparation. The preparation method comprises the following steps: mixing the following components in parts by weight: 2-32 parts of candesartan cilexetil, 10-150 parts of a diluent 1, 10-150 parts of a diluent 2, 0.5-8parts of a disintegrant, 1-10 parts of an adhesive and 0.5-10 parts of a low melting point oily compound, preparing pellets by using an extrusion and rolling method, mixing 0.05-4 parts of a lubricant with the pellets, and filling a capsule shell with the mixture, so as to obtain the capsule preparation. The capsule preparation containing candesartan cilexetil, which is disclosed by the invention, is prepared by using an extrusion and rolling preparation process and has good dissolution performance, and the bioavailability of candesartan cilexetil of the capsule preparation is greatly improved when being compared with that of a candesartan cilexetil preparation in the market.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Hypotensive tablet containing sodium chloride medicine carrier
ActiveCN103006598AReduce moisture contentReduce humidityOrganic active ingredientsInorganic non-active ingredientsPolythylene glycolPharmaceutical medicine
The invention relates to a hypotensive tablet containing a sodium chloride medicine carrier, which is suitable for a pharmaceutical enterprise. The disclosed tablet comprises the sodium chloride, candesartan cilexetil, and pharmaceutically acceptable adjuvants. The sodium chloride is stable in property and good in water solubility and compressibility, and is well compatible with the candesartan cilexetil. The sodium chloride is taken as the carrier for the candesartan cilexetil; by injecting polyethylene glycol 6000 solution or polyethylene glycol 8000 solution, a medicine is loaded on the sodium chloride and the mixed carrier containing other pharmaceutically acceptable adjuvants of the medicine; the adjuvants, such as a lubricant, are added to mix uniformly, and the mixture is subjected to tabletting and can be molded by compression under a pretty low pressure; and, by reducing the pressure in the tabletting process, the transformation of the candesartan cilexetil to an unstable crystal form due to over-high pressure in the tabletting process can be prevented so that the reduction of the candesartan cilexetil content and the increasing of the impurity content in the storage process can be prevented. The candesartan cilexetil tablet prepared by the invention has good stability and is released quickly.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
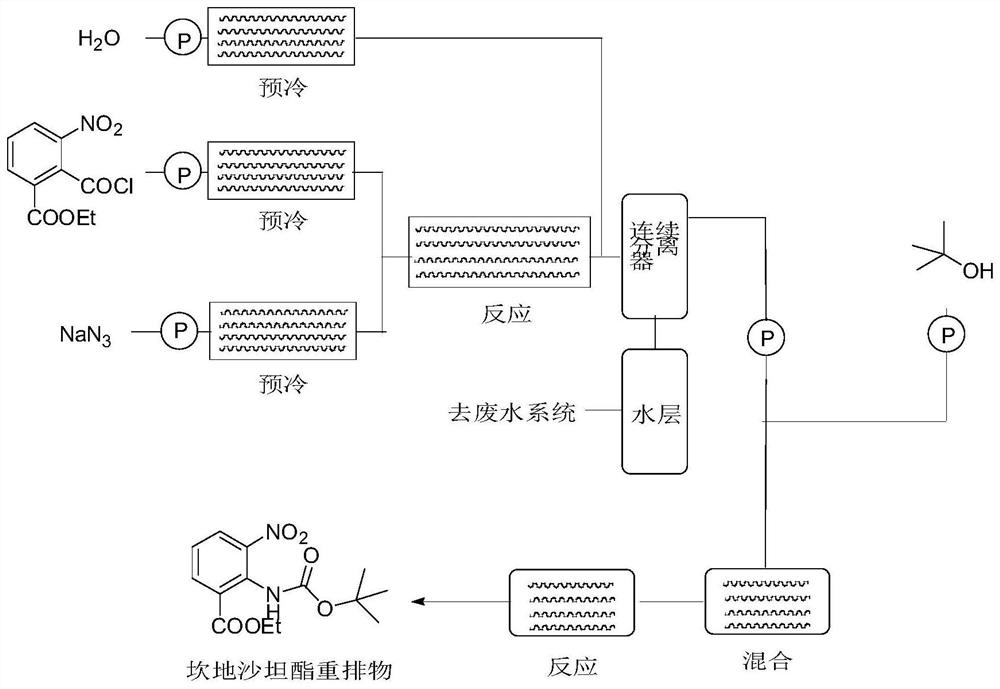
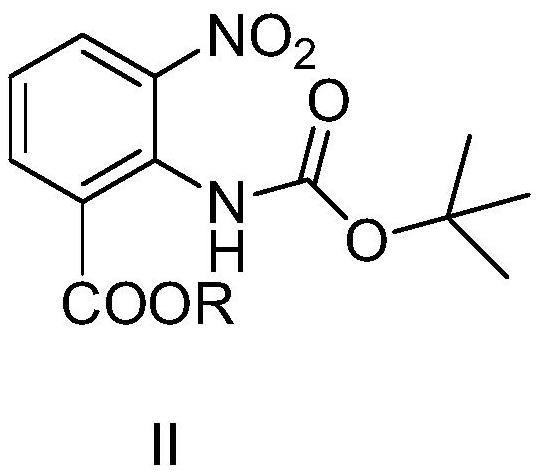
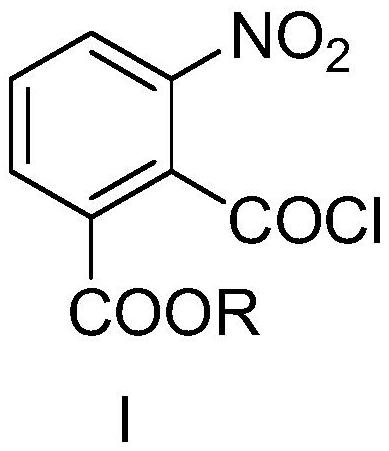
Method for synthesizing candesartan cilexetil intermediate
PendingCN113929597AEliminate huge security risksReduce consumptionSequential/parallel process reactionsCarbamic acid derivatives preparationBenzoic acidOrganic layer
The invention relates to a continuous-flow production process for synthesizing a candesartan cilexetil intermediate, belonging to the technical field of fine chemical engineering. The invention particularly relates to a process for continuously preparing 2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate by using a microchannel reactor. The process comprises the following steps: carrying out staying reaction on 2-(chlorocarbonyl)-3-nitrobenzoate and sodium azide in a first reaction module; then allowing reaction liquid and water to simultaneously enter a continuous separator for separation; and subjecting an organic layer obtained through separation to reacting with tert-butyl alcohol in a second reaction module to obtain a target product. The method provided by the invention is safe, simple to operate, efficient and easy for large-scale production.
Owner:LINHAI HUANAN CHEM CO LTD +2
Candesartan cilexetil compound and novel preparation method thereof
InactiveCN102070617AHigh purityImprove product qualityOrganic chemistryCardiovascular disorderHypertension medicationsActivated carbon
The invention provides a candesartan cilexetil compound and a novel preparation method thereof. Through absorption of active carbon, crystallization and adsorptive separation of macroporous absorption resin, the purpose of refined purification is achieved, and finally, the high-purity candesartan cilexeti is obtained, thereby greatly improving the purity and content of the candesartan cilexeti, improving the product quality of the preparation, reducing toxic and side effects and guaranteeing the clinical pharmacy safety of the candesartan cilexeti in preparing anti-hypertension medicaments. The novel preparation method has the advantages of simple process, low cost and high yield, and is suitable for industrial production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Candesartan cilexetil intermediate and application thereof
ActiveCN111747902AAvoid formingMild reaction conditionsOrganic chemistry methodsBulk chemical productionOXALIC ACID DIHYDRATEActive ingredient
The invention provides a candesartan cilexetil intermediate and a preparation method thereof. The intermediate is synthesized through the following route. The intermediate is synthesized, so that theformation of many byproducts in the reduction step in the prior art is effectively avoided, oxalic acid salifying crystallization is adopted for diamine purification in the subsequent step of synthesizing candesartan cilexetil through the intermediate, and a high-quality candesartan cilexetil bulk drug can be obtained.
Owner:ZHEJIANG TIANYU PHARMA
Candesartan cilexetil self-microemulsion soft capsule and preparation method thereof
InactiveCN102018684BImprove bioavailabilityOvercoming the problem of low bioavailabilityOrganic active ingredientsCapsule deliverySoftgelAlcohol
The invention relates to a candesartan cilexetil self-microemulsion soft capsule and a preparation method thereof. The soft capsule comprises capsule liquid and content medicine liquid. The preparation method is as follows: 100-130 parts of gelatin is arranged in mixing solution for swelling, wherein the mixing solution is formed by 18-30 parts of glycerol, 20-30 parts of sorbic alcohol and 100-120 parts of water; then 0.1-0.3 part of ethylparaben is added, heating is carried out until the gelatin is dissolved, the mixture is stirred uniformly and is filtered, and vacuum defoaming and heat insulation are carried out, thus obtaining the capsule liquid; 1-2 parts of candesartan cilexetil, 20-40 parts of assistant emulsifiers, 10-30 parts of oil phase and 80-100 parts of emulsifiers are mixed uniformly, and then the mixture is heated and stirred until the uniform and transparent content solution is formed; and the prepared capsule liquid and the prepared content solution the weight percentage ratio of which is 4:3 are moulded and pressed into soft capsules by rotating a mould press, and then drying and surface treatment are carried out on the soft capsules, thus obtaining the finished products. In the invention, the bioavailability of the candesartan cilexetil is improved, and the preparation method is simple relatively, the production is easy, thus the application prospect is wide.
Owner:HUBEI UNIV OF TECH
Candesartan cilexetil and hydrochlorothiazide co-amorphous substance and preparation method thereof
ActiveCN111888361AImprove solubilityIncrease dissolution ratePowder deliveryOrganic active ingredientsHydrochlorothiazideActive ingredient
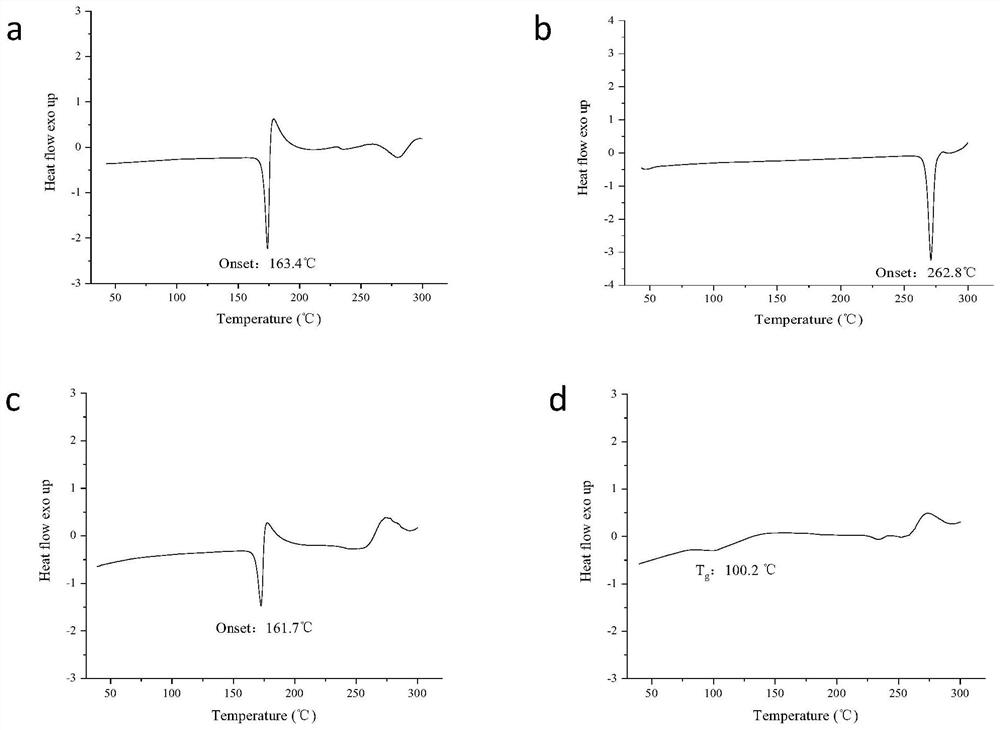
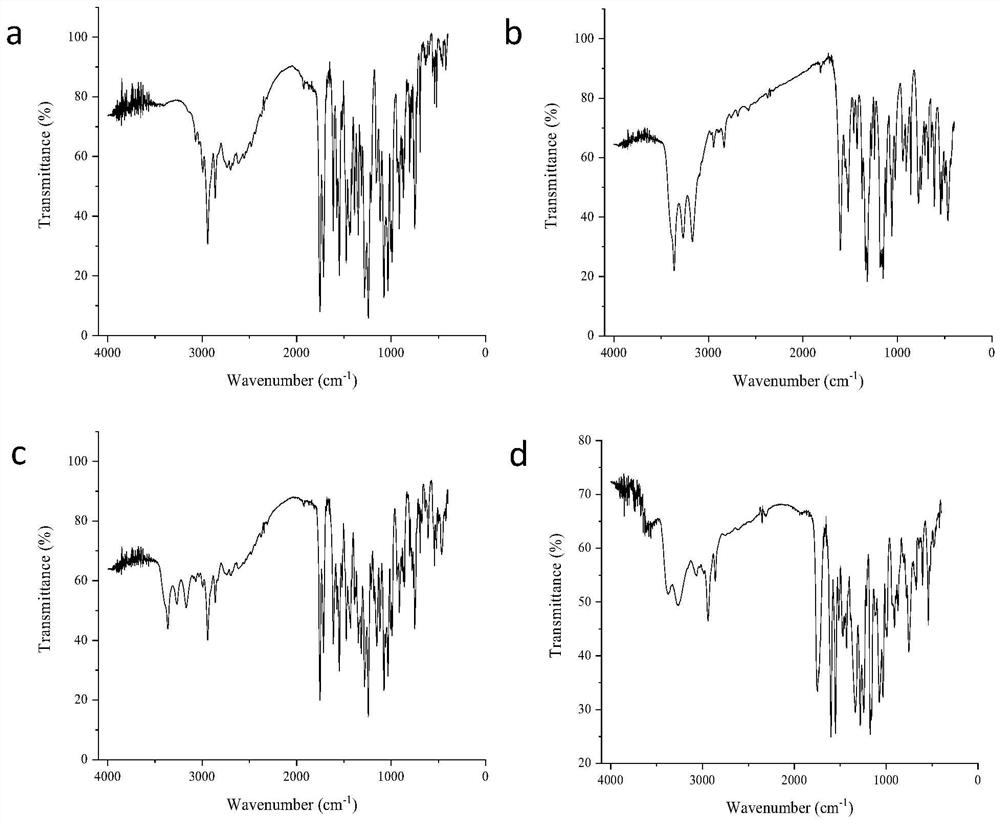
The invention discloses a candesartan cilexetil and hydrochlorothiazide co-amorphous substance formed by combining candesartan cilexetil and hydrochlorothiazide. According to a powder X-ray diffraction spectrum, a DSC spectrum and an infrared spectrum, the co-amorphous substance is a novel solid form completely different from each monomer and a physical mixture thereof. The candesartan cilexetil and the hydrochlorothiazide are used for preparing the co-amorphous substance, so that the solubility and the dissolution rate of the candesartan cilexetil can be significantly improved, and the co-amorphous substance is expected to become a new raw material solid form of a candesartan cilexetil / hydrochlorothiazide compound tablet and has good application development prospects.
Owner:CHINA PHARM UNIV
Method for preparing candesartan cilexetil
ActiveCN101781286BReduce usageHigh yieldOrganic chemistryBulk chemical productionBenzoic acidTetrazole
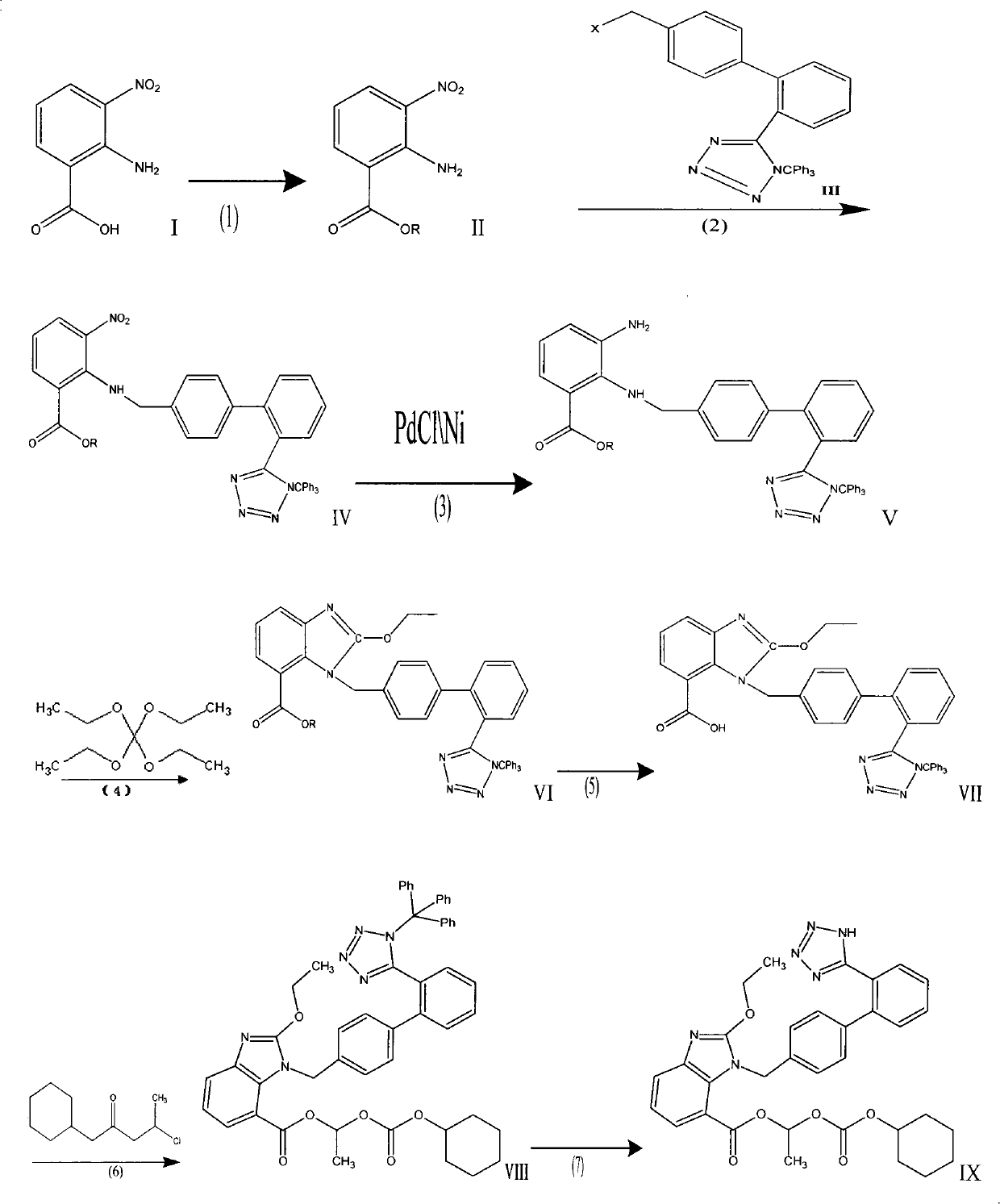
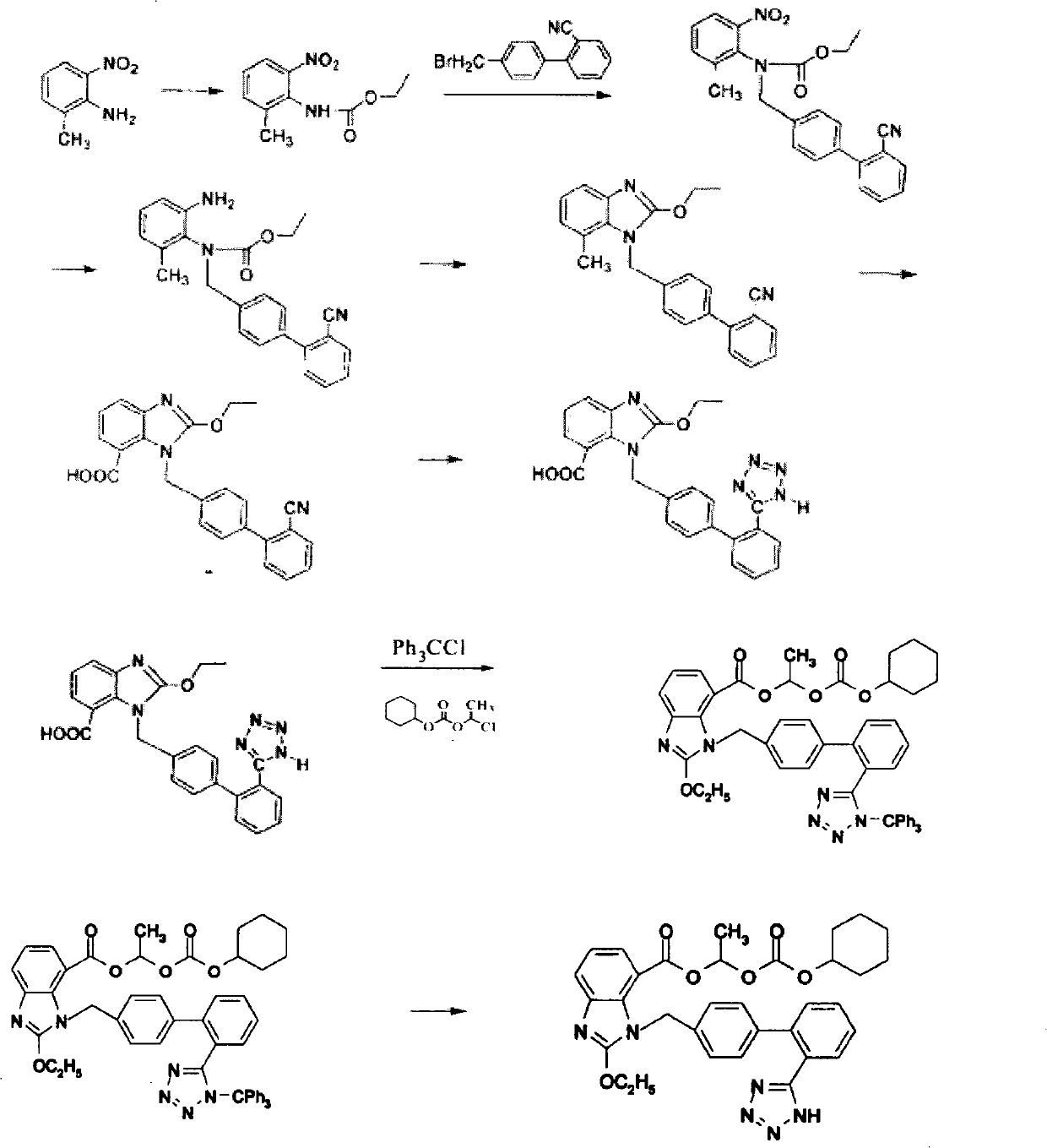
The invention provides a method for preparing candesartan cilexetil, which can solve the problems of longer reaction route, easy remaining of toxic substances in medicines and lower total yield existing in the prior art. In the method, the candesartan cilexetil is finally prepared by using 2-amino-3-nitrobenzoic acid as an initial raw material through esterification reaction, N-alkylation reaction, nitro reduction reaction, cyclization reaction, hydrolysis reaction, esterification reaction and tetrazole protecting group deprotection reaction. The synthetic method has simple steps and high yield, greatly reduces the participation and generation of toxic products in the reaction process, reduces the release of waste and is beneficial to clean production.
Owner:QINGDAO HUANGHAI PHARM CO LTD
A kind of detection method of genotoxic impurity of candesartan cilexetil
ActiveCN110501449BEffective controlReduce drug side effectsComponent separationCarbonate esterActive ingredient
The invention relates to a method for analyzing the genotoxic impurity 1-chloroethylcyclohexyl carbonate of candesartan cilexetil, belonging to the technical field of crude drug analysis. The present invention utilizes the impurity 1-chloroethyl cyclohexyl carbonate existing in candesartan cilexetil and the pre-column derivation method of the derivation reaction with candesartan cilexetil itself to measure the impurity 1-chloroethyl cyclohexyl carbonate content, to achieve the control of genotoxic impurity 1-chloroethyl cyclohexyl carbonate in candesartan cilexetil. The invention provides a detection method for the genotoxic impurity in candesartan cilexetil, which provides guarantee for the quality control of candesartan cilexetil preparations.
Owner:迪嘉药业集团股份有限公司
A new oral solid pharmaceutical composition and its preparation method
ActiveCN102342942BHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryCaplet Dosage FormLevamlodipine
Owner:HAINAN JINRUI PHARMA CO LTD
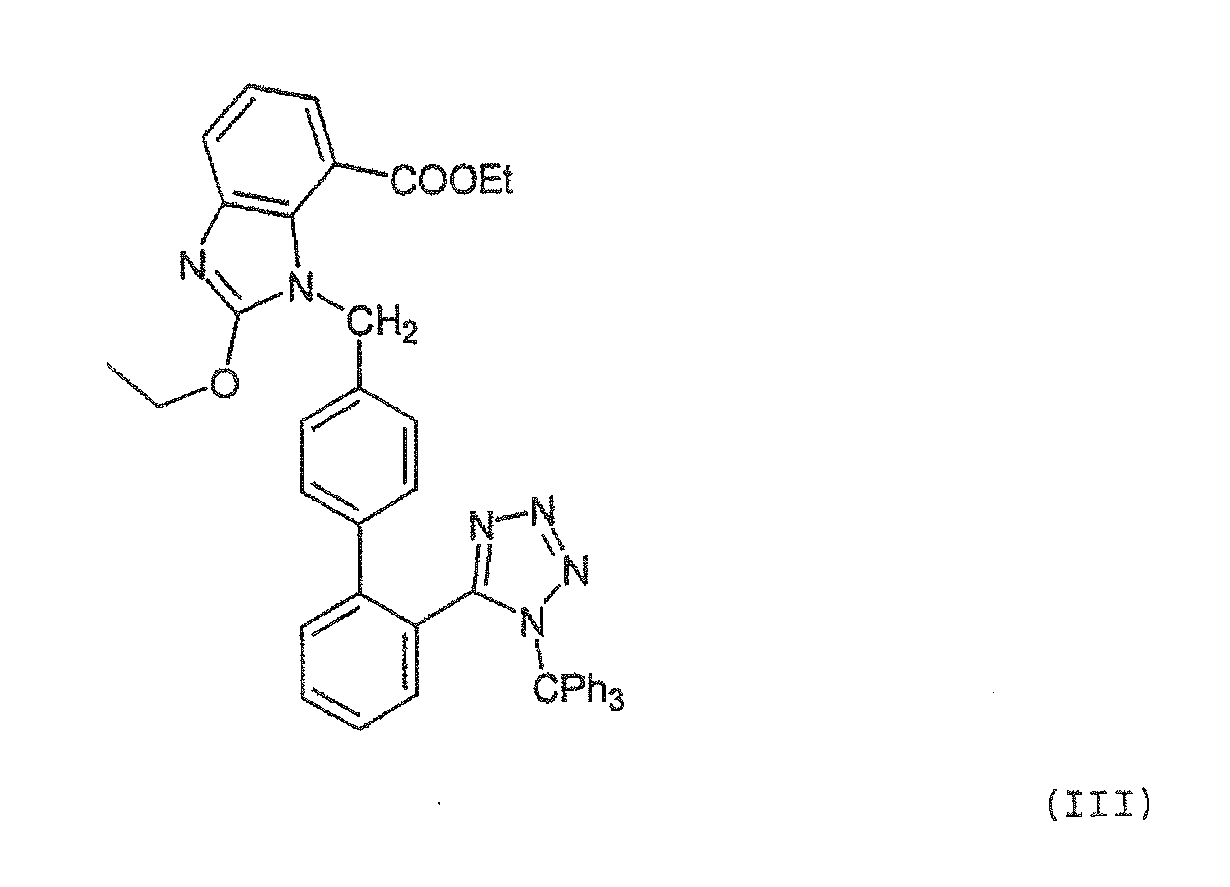
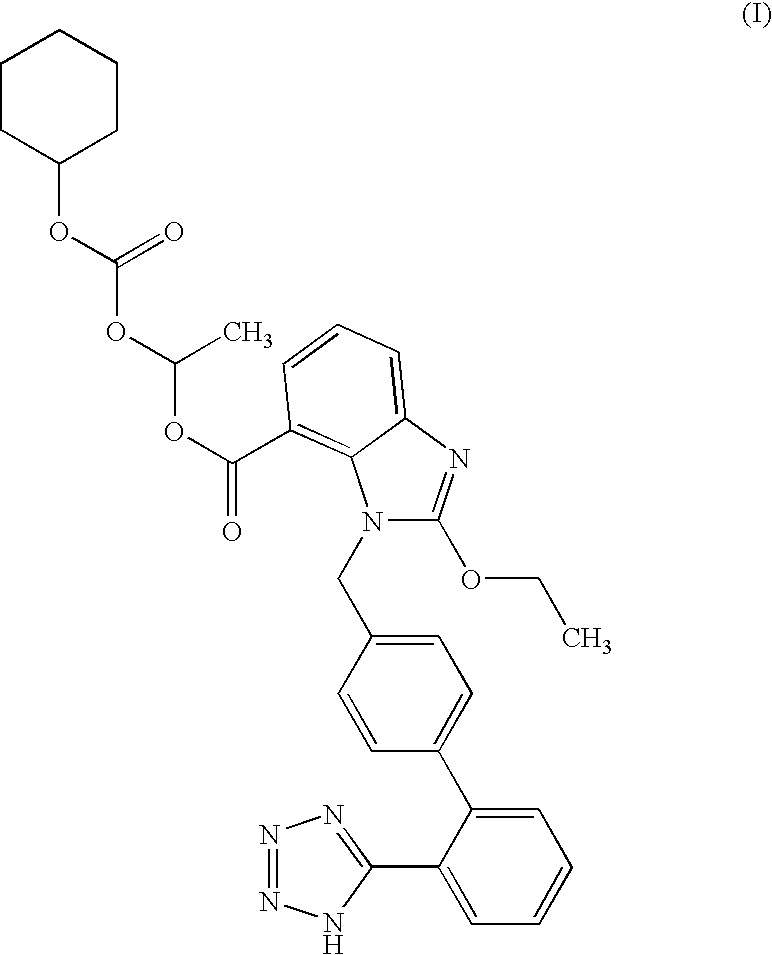
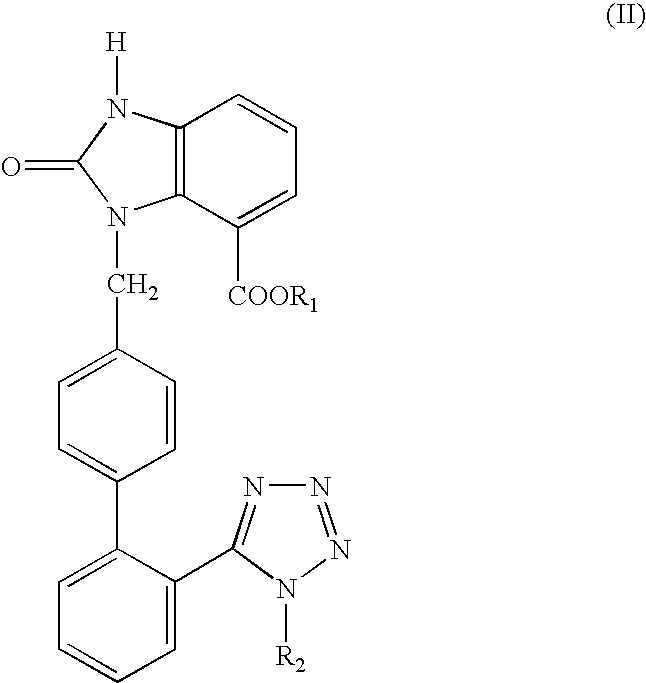
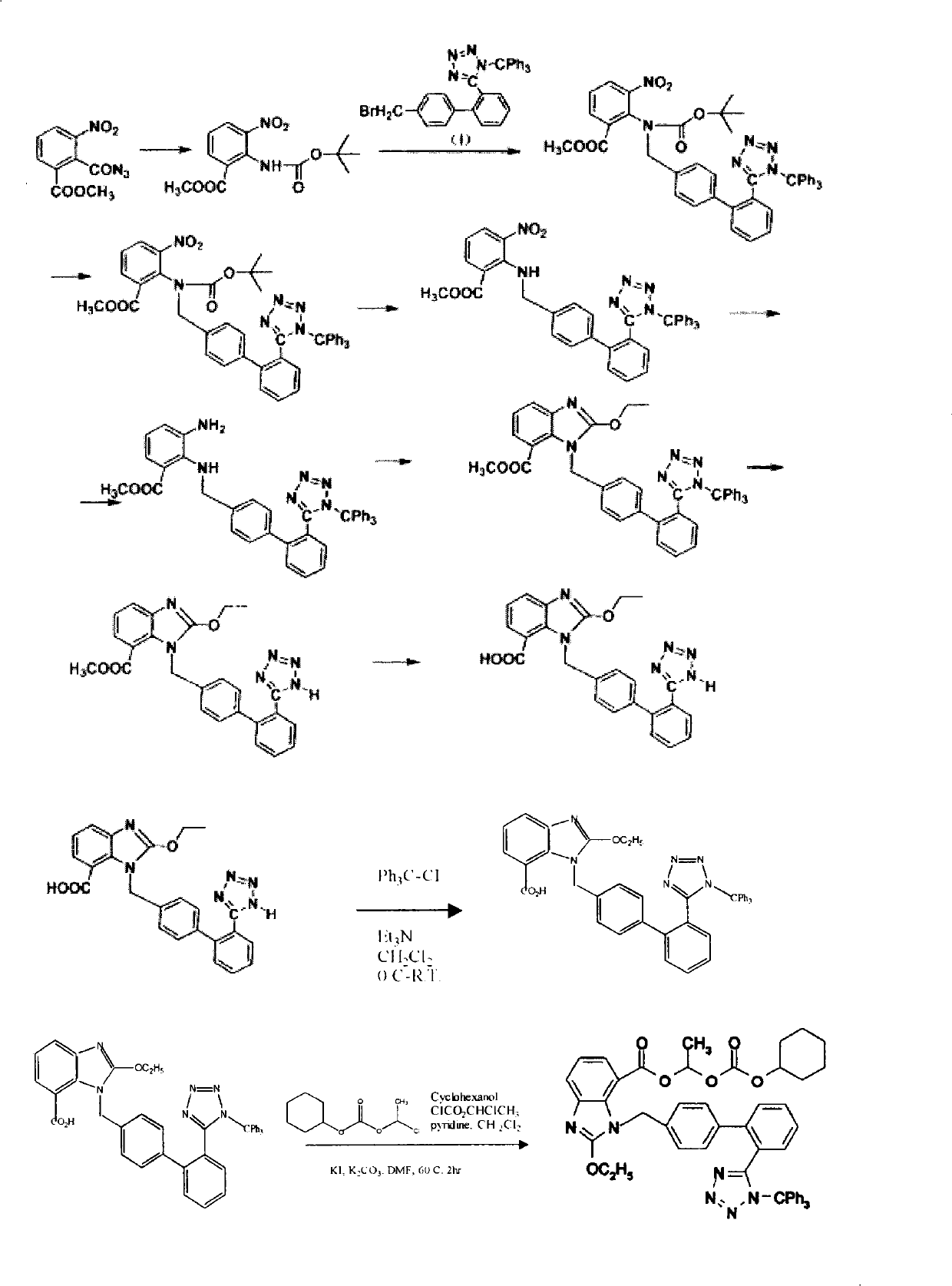
Candesartan Cilexetil and precursor compound preparation method thereof
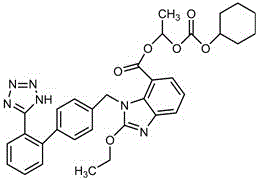
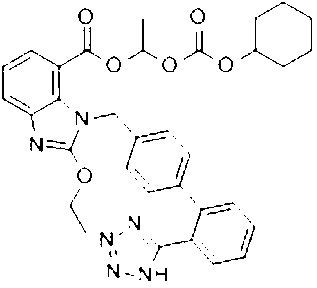
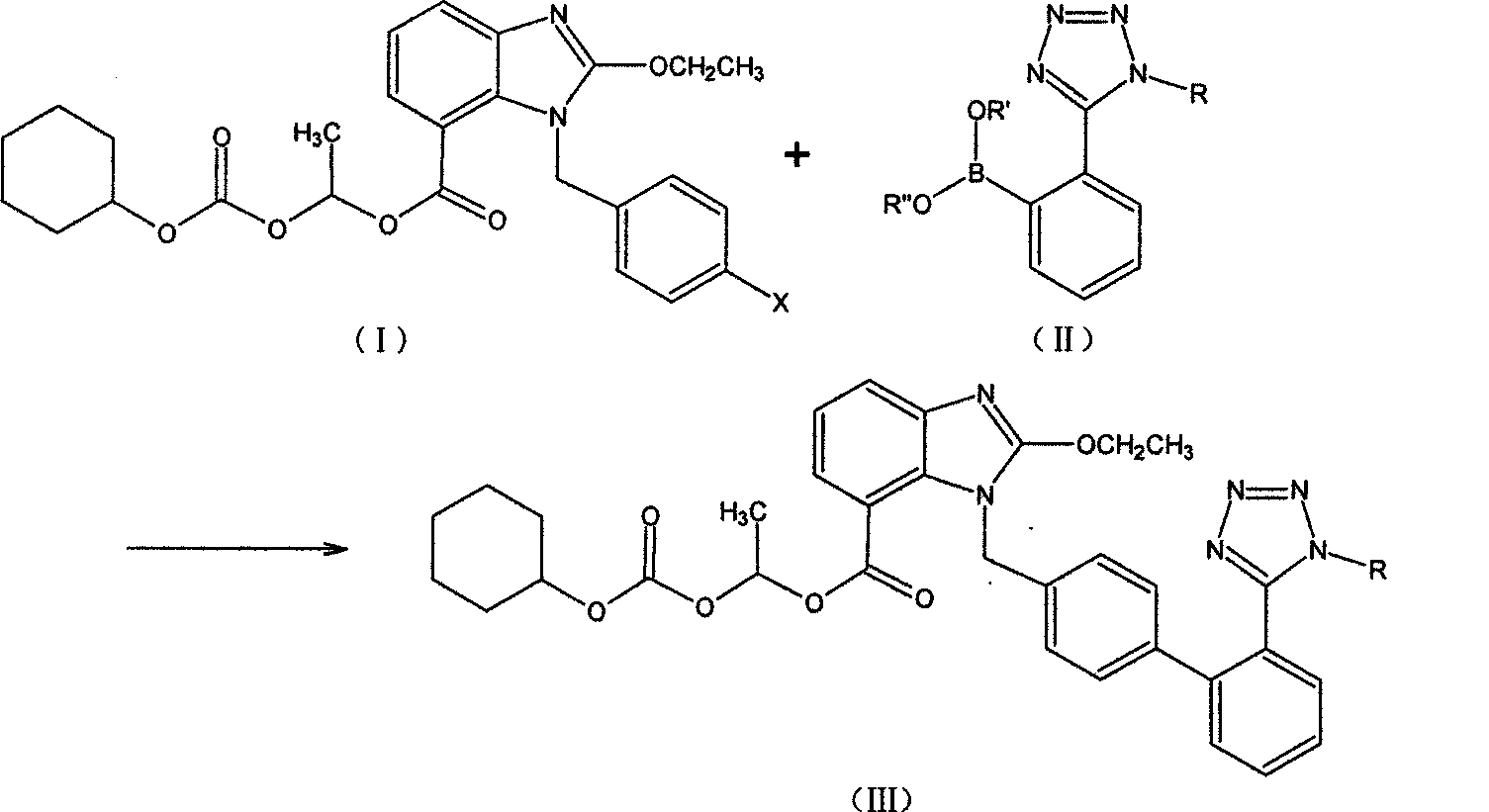
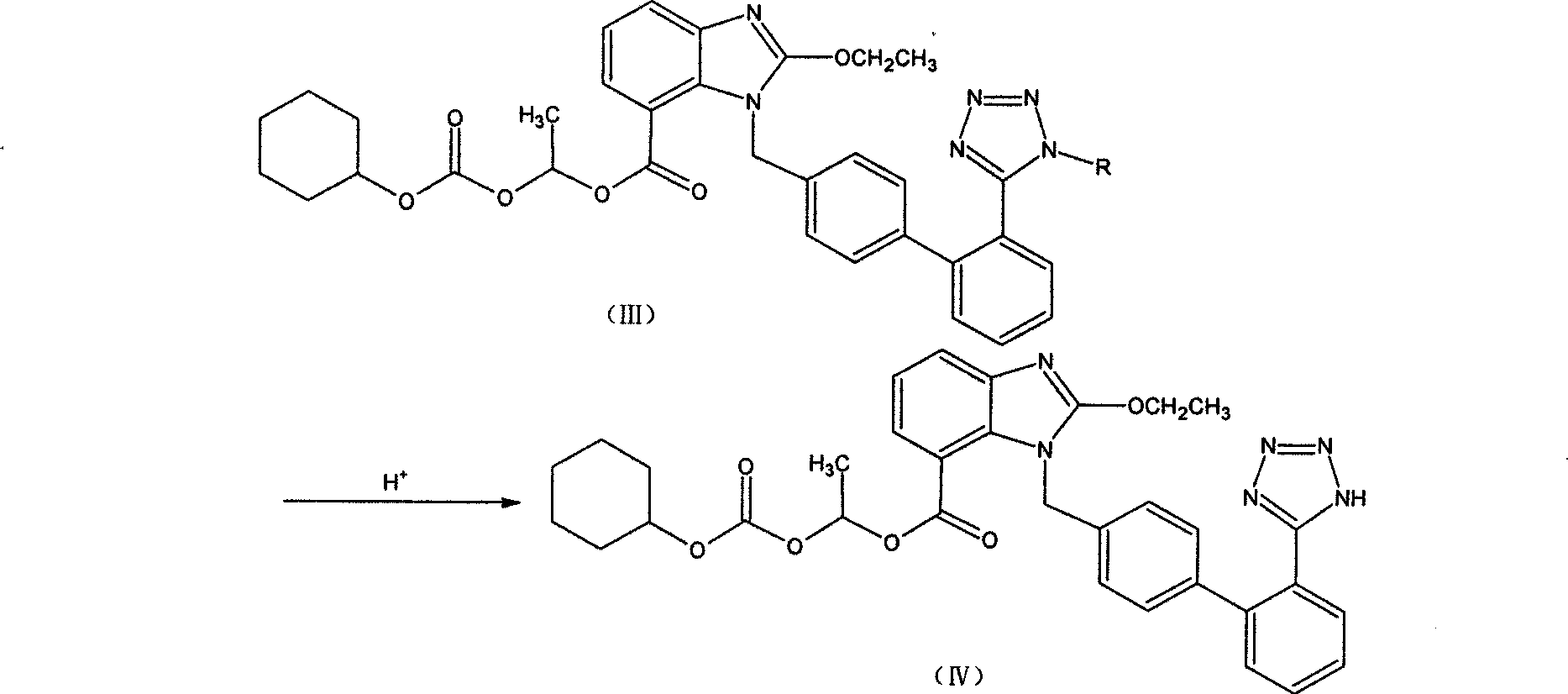
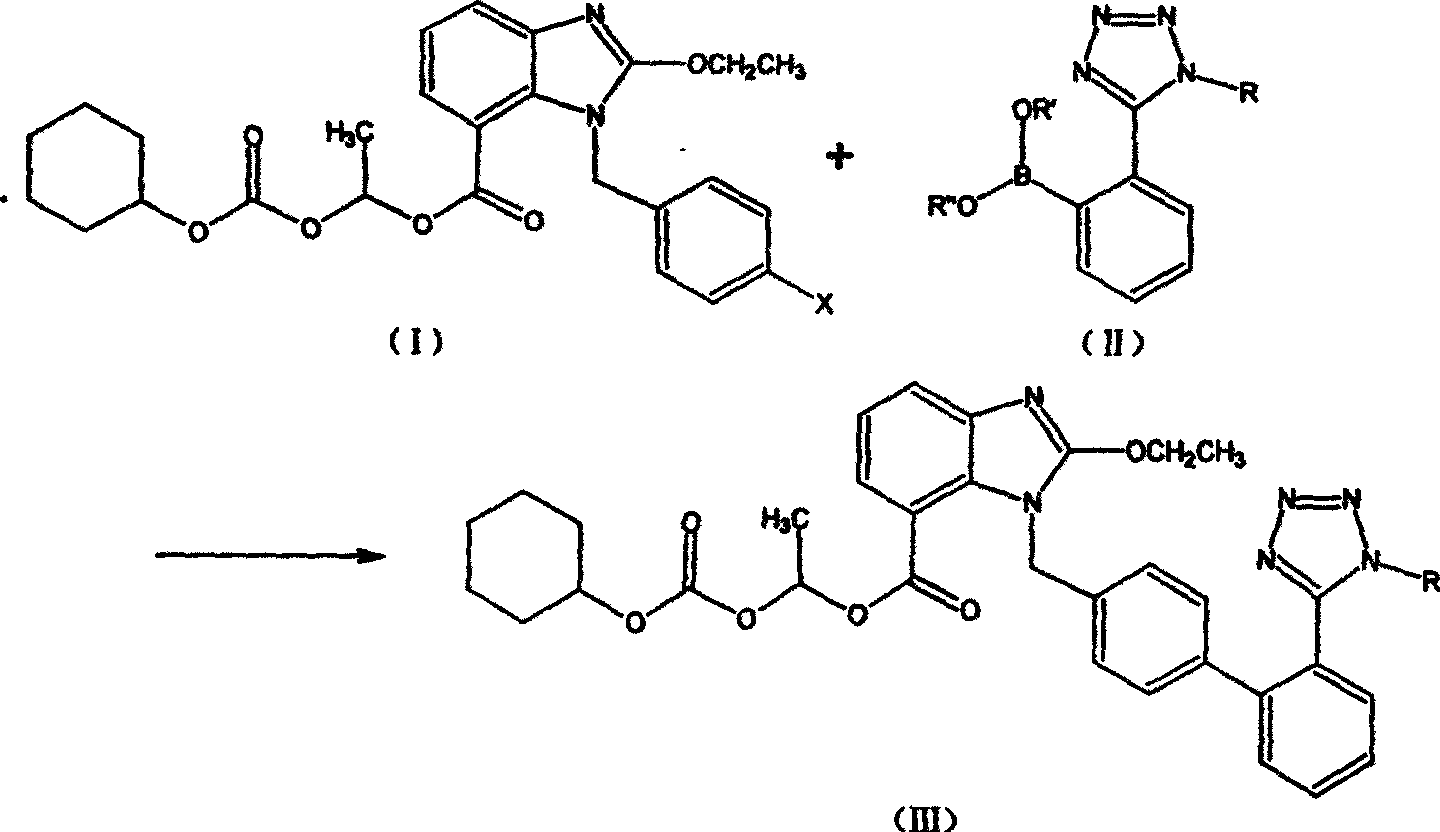
The preparation method of candesartan cilexetil and its precursor compound. The precursor compound is 2-ethoxy-1-[[2′-(1-R-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H -Benzimidazole-7-carboxylic acid 1-(cyclohexyloxycarbonyloxy)ethyl ester (III), from 2-ethoxy-1-(4'-halophenyl)methyl-1H-benzene Imidazole-7-carboxylic acid 1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester (I) and 2-(1-R-1H-tetrazol-5-yl)phenylboronic acid or 2- (1-R-1H-tetrazol-5-yl) phenyl borate (II) reacts in a soluble organic reaction medium and in the presence of a catalyst and a water-soluble basic compound allowed by the Suzuki reaction get. The precursor compound can be further hydrolyzed under acidic conditions to obtain candesartan cilexetil which can be used as medicine. The method can conveniently and advantageously prepare the candesartan cilexetil compound.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Fortifier
ActiveCN1784235AExcellent inhibitory effect on nephropathy progressionEnhanced inhibitory effect on kidney disease progressionOrganic active ingredientsOrganic chemistryNephrosisProstaglandins I
The invention discloses a nephropathy treating or preventing effect enhancer of candesartan cilexetil and other renin-angiotensin system inhibitors. The enhancer contains specific prostaglandin I derivatives such as bereprosodium as active ingredients.
Owner:TORAY IND INC
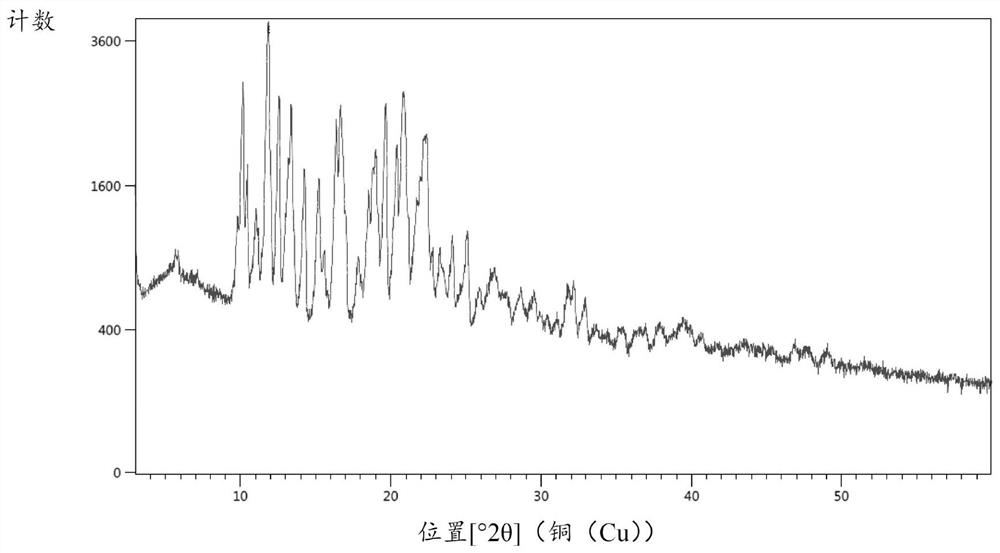
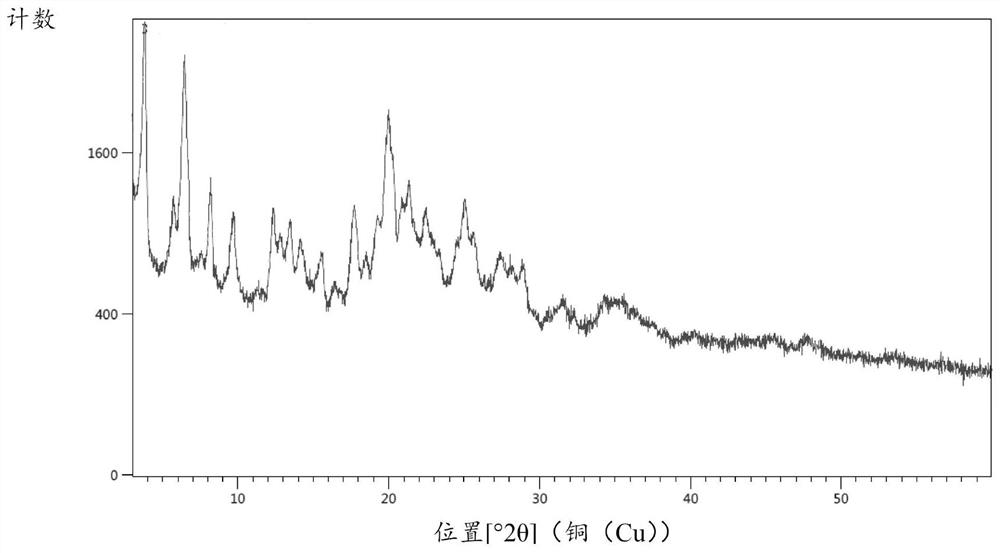
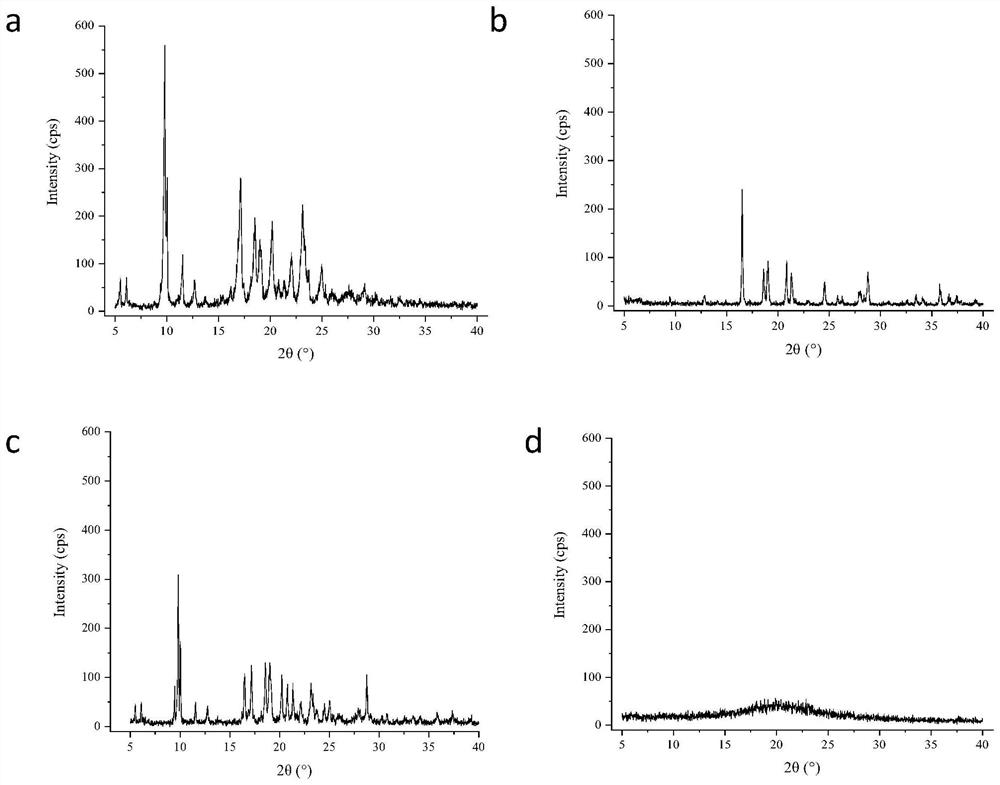
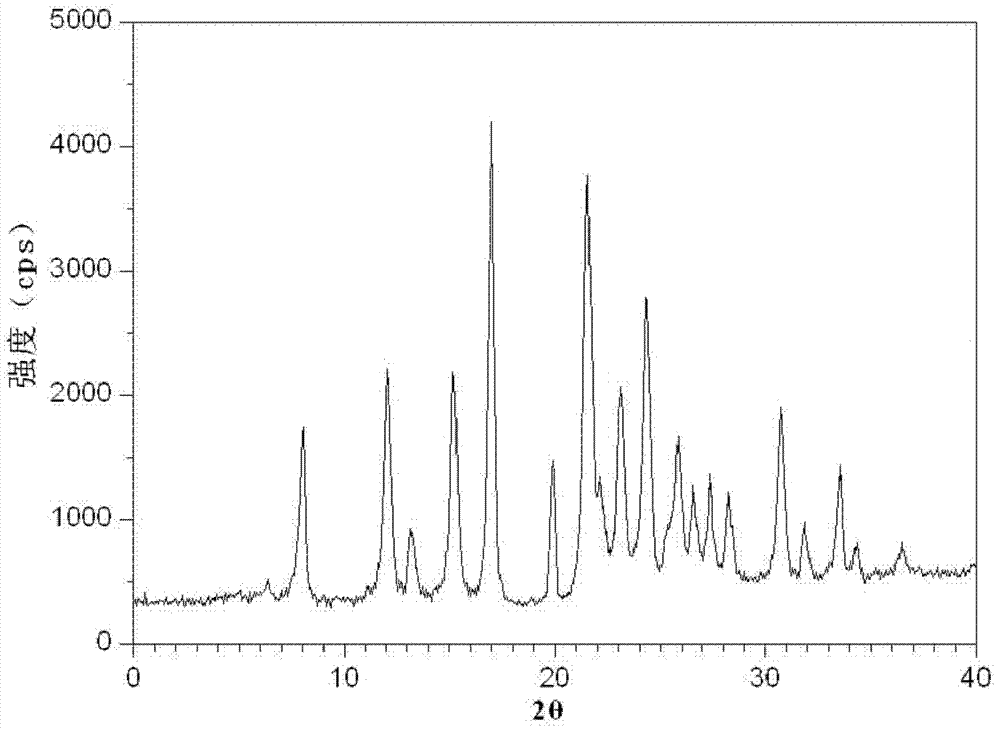
Hydrochlorothiazide crystal and candesartan cilexetil hydrochlorothiazide medicinal combination thereof
The invention discloses a hydrochlorothiazide crystal and a candesartan cilexetil hydrochlorothiazide medicinal combination thereof. The characteristic peaks of the hydrochlorothiazide crystal in an X-ray powder diffraction pattern, which are measured and obtained by using a Cu-K[alpha] ray, are displayed when 2[theta] is 4.1 degrees, 8.2 degrees, 9.8 degrees, 12.1 degrees, 15.1 degrees, 16.7 degrees, 19.3 degrees, 20.0 degrees, 22.1 degrees, 23.3 degrees and 26.8 degrees. The combination comprises 4-20 parts of candesartan cilexetil, 10-15 parts of the hydrochlorothiazide crystal, 10-50 parts of pregelatinized starch, 15-35 parts of microcrystalline cellulose PH102, 10-45 parts of crosslinked polyvinylpyrrolidone and 0.5-1 part of magnesium stearate. The medicinal combination has a reasonable prescription, stable and reliable quality, and better disintegration time limit and dissolution rate; a direct powder tabletting process is adopted; the process is simple; the production period is short; the production cost is low; and the industrialized production is facilitated.
Owner:HAINAN JINRUI PHARMA CO LTD
The detection method of a nitrodite compound in Kandetartin
ActiveCN110514759BQuick and easy separation and quantitative determinationQuality improvementComponent separationSilanesSodium azide
The invention relates to the technical field of chemical drug analysis, in particular to a method for detecting azide compounds in candesartan cilexetil. The detection method of the present invention is to take candesartan cilexetil raw material drug to be tested, add acetonitrile for ultrasonic dissolution, and then dilute with sulfuric acid aqueous solution; the volume ratio of the acetonitrile and sulfuric acid water is 40:60~60:40; after fully shaking Filtrate, get the filtrate and stand-by as need testing solution; Chromatographic column uses octadecylsilane bonded silica gel as stationary phase, adopts acetonitrile-sulfuric acid aqueous solution as mobile phase A, acetonitrile-water as mobile phase B to carry out gradient elution, carry out HPLC detection, record spectrum. The detection method of the invention can quickly and conveniently separate and quantitatively determine the sodium azide in the candesartan cilexetil, thereby effectively controlling the quality of the candesartan cilexetil product.
Owner:HINYE PHARM CO LTD
Brand new drug composition containing levamlodipine besylate and candesartan cilexetil and preparation method thereof
ActiveCN102349902BImprove solubilityRapid dissolutionOrganic active ingredientsLevamlodipinePharmaceutical medicine
Owner:HAINAN JINRUI PHARMA CO LTD
Method for recovering candesartan cilexetil intermediate mother liquor
ActiveCN113444009AReduce manufacturing costAvoid interferenceIsocyanic acid derivatives preparationOrganic compound preparationMedicinal chemistryPharmacology
The invention provides a recovery method of candesartan cilexetil intermediate mother liquor. The candesartan cilexetil intermediate mother liquor obtained in real production comprises candesartan tert-butyl ester, candesartan tert-butyl ester decomposition impurities and candesartan tert-butyl ester diester impurities; and the mother liquor is not treated in conventional production procedures and is directly treated as waste liquor, so resources are wasted and cost is increased. The method for recovering the mother liquor provided by the invention can recover candesartan cilexetil in the mother liquor, convert decomposed impurities and diester impurities into candesartan cilexetil for recovery, and recover more than 90% of substances in the mother liquor, so the production cost of candesartan cilexetil is greatly reduced. According to the recovery method, interference of diester impurities can be avoided when the decomposed impurities are recycled, and generation of byproduct diester impurities is avoided as much as possible when the diester impurities are recycled; and operation is easy and convenient, and raw materials are low in price, so the method is suitable for industrial production and popularization.
Owner:珠海润都制药股份有限公司
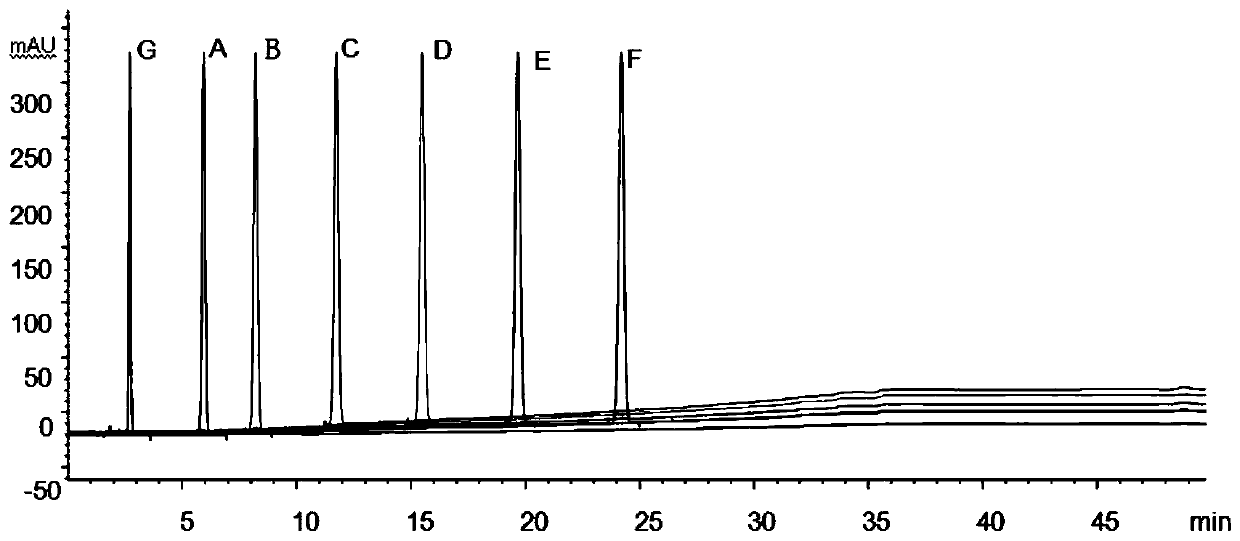
Method for determining impurities in candesartan cilexetil
ActiveCN111458444ADoes not affect positioningGuaranteed separation effectComponent separationFluid phasePhysical chemistry
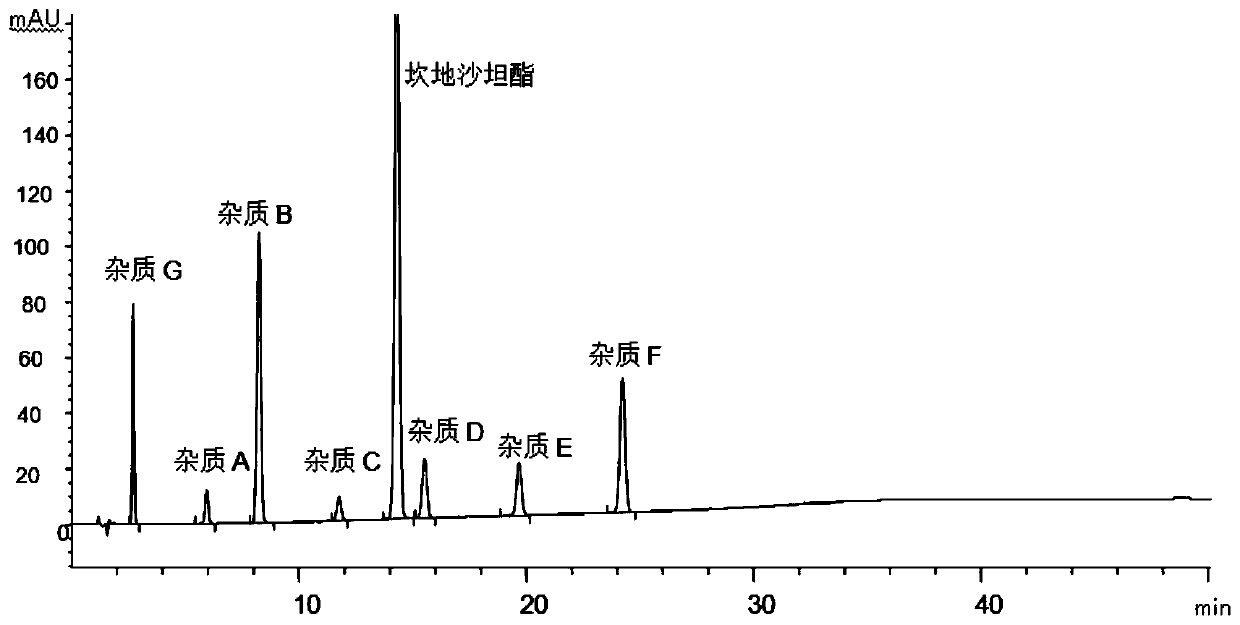
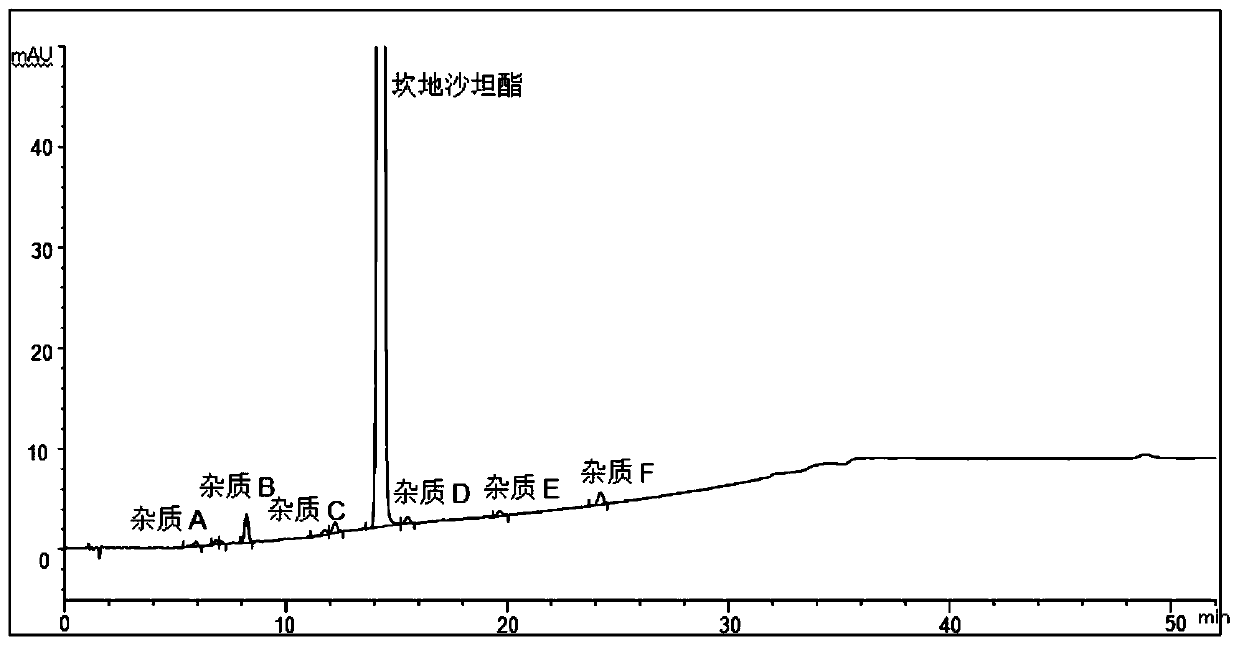
The invention discloses a method for determining impurities in candesartan cilexetil. The method comprises the following steps: S1, heating a candesartan cilexetil standard substance solution at 70-100 DEG C for 1.5-3 hours, cooling, and adding acetonitrile to obtain a degradation solution 1; besides, adding the candesartan cilexetil standard substance solution into an alkaline solution, performing standing for 5-30 minutes, and adding an acid solution for neutralization to obtain a degradation solution 2; mixing the degradation solution 1 and the degradation solution 2 to obtain a system applicability solution; S2, dissolving candesartan cilexetil to be detected, and quantitatively preparing a test solution; diluting the test solution as a control solution; and S3, respectively carrying out liquid chromatography detection on the system suitability solution obtained in the step S1, the test solution obtained in the step S2 and the control solution, and determining the impurities A, B,C, D, E, F or G in candesartan cilexetil by comparing liquid chromatograms. The method is strong in pertinence, high in accuracy and easy to popularize, and has good application value in the aspect ofdetermining the impurities in candesartan cilexetil.
Owner:广东省药品检验所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com