Candesartan cilexetil intermediate and application thereof
A technology of candesartan cilexetil and intermediates, applied in the field of candesartan cilexetil intermediates, can solve the problems of many side reactions, difficult to remove impurities, unsatisfactory effects, etc., achieves mild reaction conditions, avoids by-products, converts high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of intermediate compound 1
[0049]
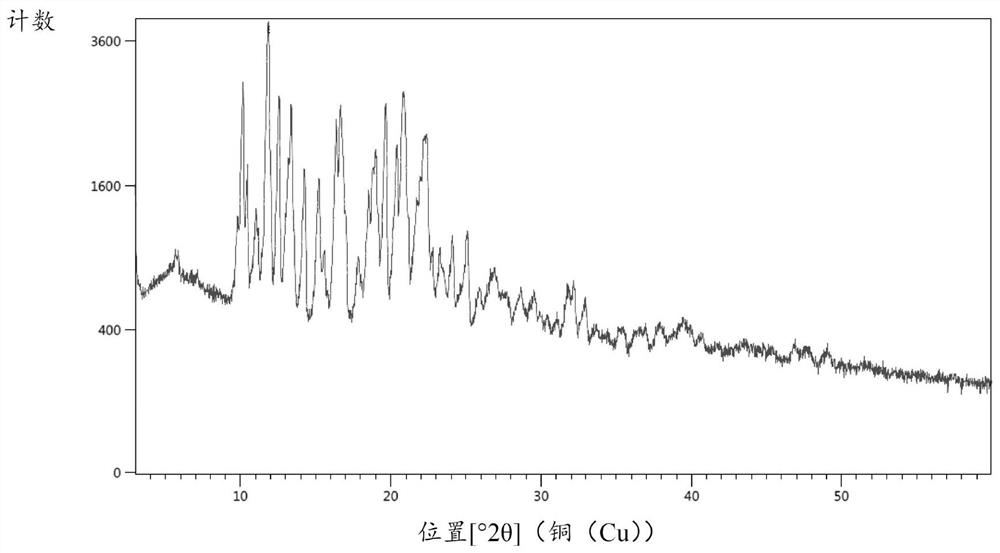
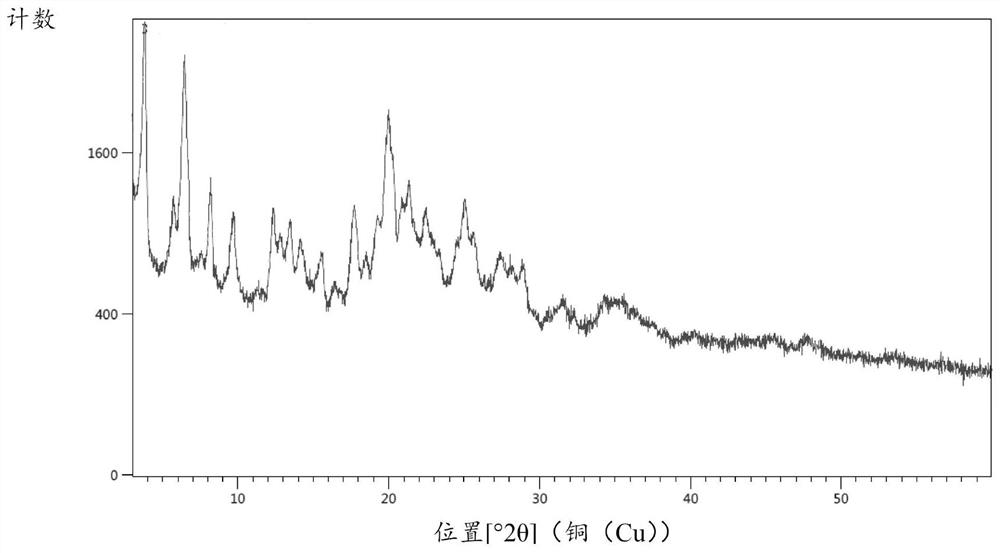
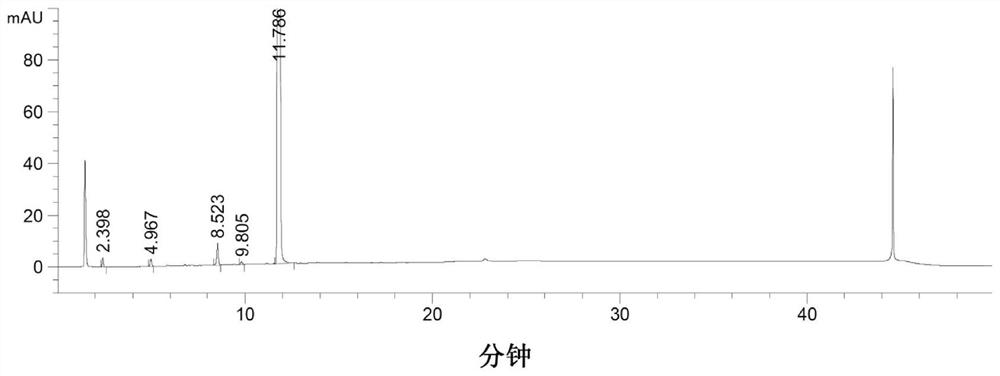
[0050] Put 20g of compound 2, 35.5g of tetrahydrofuran, and 2.4g of activated carbon into the reaction flask, add 1.2g of ferric oxyhydroxide under stirring, then slowly heat up to 40-45°C, and drop 5.8g of hydrazine hydrate (80%w / w), the dropwise addition is completed, and the reaction is kept at 40-45°C for 20-30 hours, and the TLC spot plate is controlled (ethyl acetate:n-hexane=4:5, V / V). After the reaction is completed, the synthetic solution is reduced to 20-25°C, filter, rinse the filter cake with 20g of tetrahydrofuran, combine the filtrates, precipitate the filtrate to dryness, and recrystallize the residue with 50g of ethyl acetate and 5g of water to obtain 17.7g of intermediate compound 1 crystals, with a yield of 92% , HPLC purity 98.5%, its X-ray powder diffraction pattern sees figure 1 . ESI-HRMS(m / z):C 45 h 41 N 6 o 4 [M+H +]Theoretical calculation value: 729.3184, measured val...
Embodiment 2
[0051] Embodiment 2: the preparation of compound 3
[0052]
[0053] Add 15g of compound 1, 40.05g of ethyl acetate, and 1.7g of potassium carbonate to the reaction flask, heat up to 40-45°C, add 4.7g of cyclohexyl chloroethyl carbonate dropwise, after the addition is complete, heat up to 60-65°C, and keep warm Stir for 3-5 hours, TLC point plate in the control (ethyl acetate: n-hexane = 4:5 (glacial acetic acid), V / V), reduce to 20-25 ° C, filter, add 10 g ethyl acetate to rinse the filter cake , Combine the filtrates, add 10 g of water to the filtrate to wash once, add 42 g of ethyl acetate to the ethyl acetate layer to obtain the ethyl acetate feed solution of compound 3, and the ethyl acetate feed solution of compound 3 is directly used in the next step reaction.
Embodiment 3
[0054] Embodiment 3: the preparation of oxalate compound 4
[0055]
[0056] Control the temperature of the ethyl acetate feed solution of compound 3 obtained in the previous step and stir at 10-15°C, add 20.2g of hydrobromic acid (33%w / w) dropwise, after the addition is completed, heat up to 40-45°C, Keep the reaction at 45°C for 3 hours, lower the temperature to 0-5°C, keep stirring for 1 hour, filter, add 10 g of ethyl acetate to rinse, and obtain the crude wet product of the deprotected product of compound 3. Add 60g of ethyl acetate to the crude wet product, if it is not dissolved, add aqueous sodium bicarbonate solution dropwise to adjust the pH to 6-7, dissolve it, let it stand for layering, wash the organic layer once with 10g of saturated saline, and then add 10g of anhydrous sulfuric acid Sodium, 1g activated carbon, dry at 40-45°C, decolorize for 1 hour, filter, de-dry the filtrate, add 114g acetonitrile to dissolve, add 4.4g oxalic acid, heat up to 30-35°C, keep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com