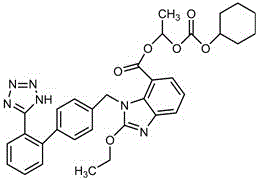
Preparation method of candesartan cilexetil crystal form I spherical crystal
A kind of candesartan medoxomil and crystallization technology, applied in the field of preparation of candesartan medoxomil crystal form I spherical crystal, can solve the problems such as no preparation method of candesartan medoxomil spherical crystal, achieve good fluidity, particle size The effect of uniform distribution, which is conducive to stable production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
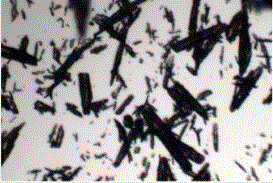
[0019] Add 10 g of candesartan cilexetil into a container containing 100 mL of 2-butanone, stir and dissolve at 20°C, and then filter and decolorize after stirring continuously for 60 minutes; transfer the filtrate to a crystallizer, control the system temperature to 10°C, Add methanol solvent for elution and crystallization, control the dropping time to 2h, and the volume of methanol used is 5 times that of 2-butanone; then filter and separate, wash the filter cake with methanol, and store the obtained product at a temperature of 40°C under 0.08MPa Drying was carried out under the condition of vacuum degree for 6h. The final product product appearance is spherical (such as figure 2 shown), the HPLC purity is 99.56%, the primary particle size is 180 microns, and the single-pass molar yield in the crystallization process is 89.7%.
Embodiment 2
[0021] Add 3 g of candesartan cilexetil into a container containing 100 mL of 2-butanone, stir and dissolve at 20°C, and then filter and decolorize after stirring continuously for 30 minutes; transfer the filtrate to a crystallizer, control the system temperature to 10°C, Add ethanol solvent for elution and crystallization, control the dropping time to 3 hours, and the volume of ethanol used is 6 times that of 2-butanone; then filter and separate, wash the filter cake with ethanol, and store the obtained product at a temperature of 60°C under 0.1MPa Drying was carried out for 5 h under the condition of vacuum degree. The appearance of the final product is spherical, the HPLC purity is 99.72%, the main particle size is 175 microns, and the single-pass molar yield in the crystallization process is 88.3%.
Embodiment 3
[0023] Add 5 g of candesartan cilexetil into a container containing 100 mL of 2-butanone, stir and dissolve at 30°C, and then filter and decolorize after stirring continuously for 40 minutes; transfer the filtrate to a crystallizer, control the system temperature to 20°C, Add isopropanol solvent to carry out elution crystallization, control drop time is 2h, isopropanol volume consumption is 4 times of 2-butanone; Carry out filtration separation then, wash filter cake with isopropanol, the product that obtains is in 50 ℃ temperature, under the condition of 0.06MPa vacuum for 5h. The appearance of the final product is spherical, the HPLC purity is 99.66%, the main particle size is 165 microns, and the single-pass molar yield in the crystallization process is 89.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com