Method for preparing candesartan cilexetil
A technology for candesartan cilexetil and esterification, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of long reaction route, drug residues, low total yield of toxic substances, etc. Yield, reducing effect of sodium azide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
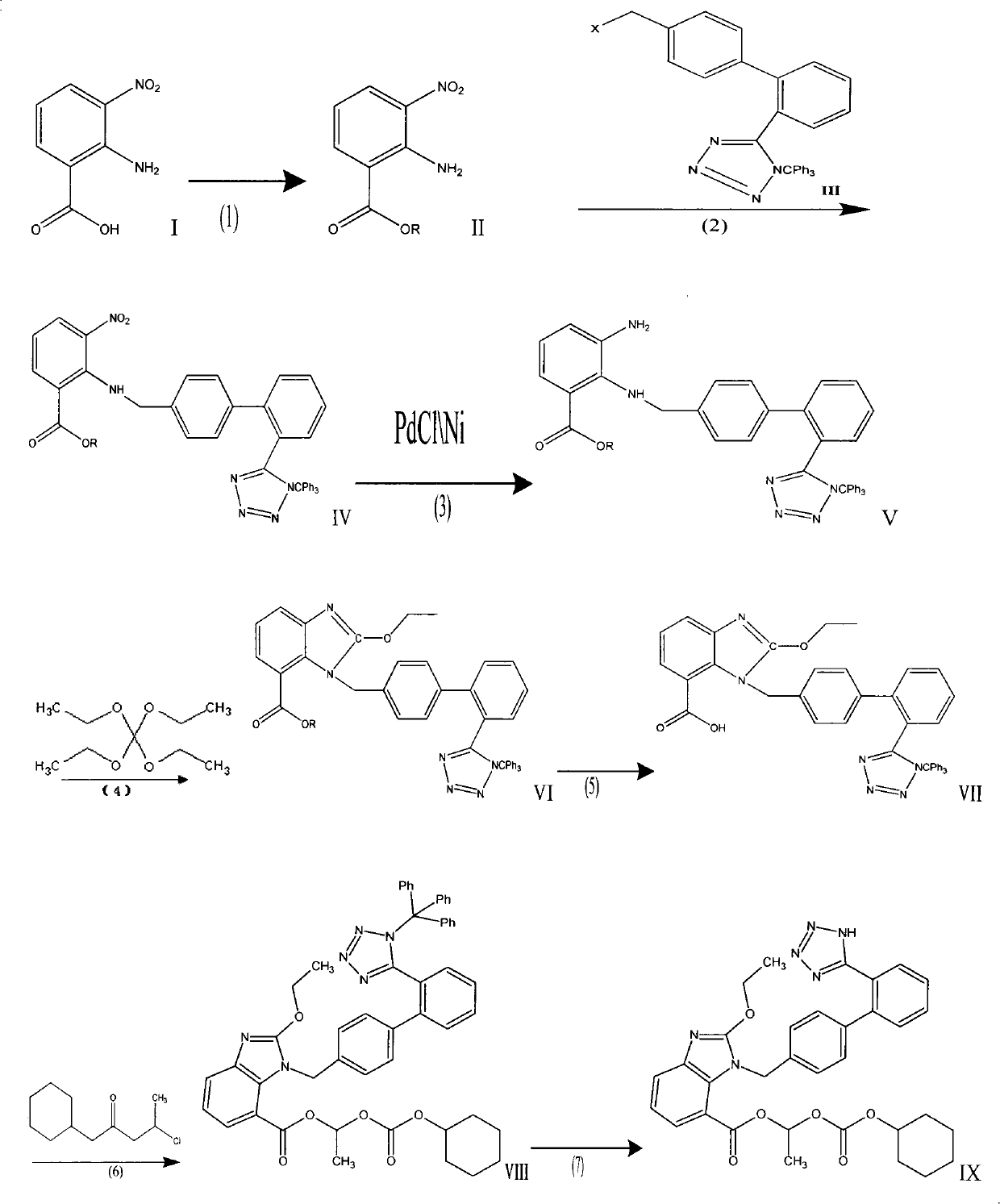
[0043] Wherein in embodiment 1: (R=methyl, X=Br)
[0044] 1, 2-amino-3-nitrobenzoic acid;
[0045] II. Methyl 3-nitro-2-aminobenzoate;
[0046] III, N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium;
[0047] IV, 2-N-[[(2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl]amino)- Methyl 3-nitrobenzoate;
[0048]V, 2-N-[[(2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl]amino)- Methyl 3-aminobenzoate;
[0049] VI, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] -1-H-benzimidazole-7-carboxylic acid methyl ester;
[0050] VII, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] -1-H-benzimidazole-7-carboxylic acid;
[0051] VIII, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] -1-(cyclohexyloxycarbonyloxy)ethyl 1-H-benzimidazole-7-carboxylate;
[0052] (1), the synthesis of methyl 3-nitro-2-aminobenzoate (II)
[0053] Add 18.2g (0.1mol) of 3-nitro-2-aminobenzoic acid into the reac...
Embodiment 2
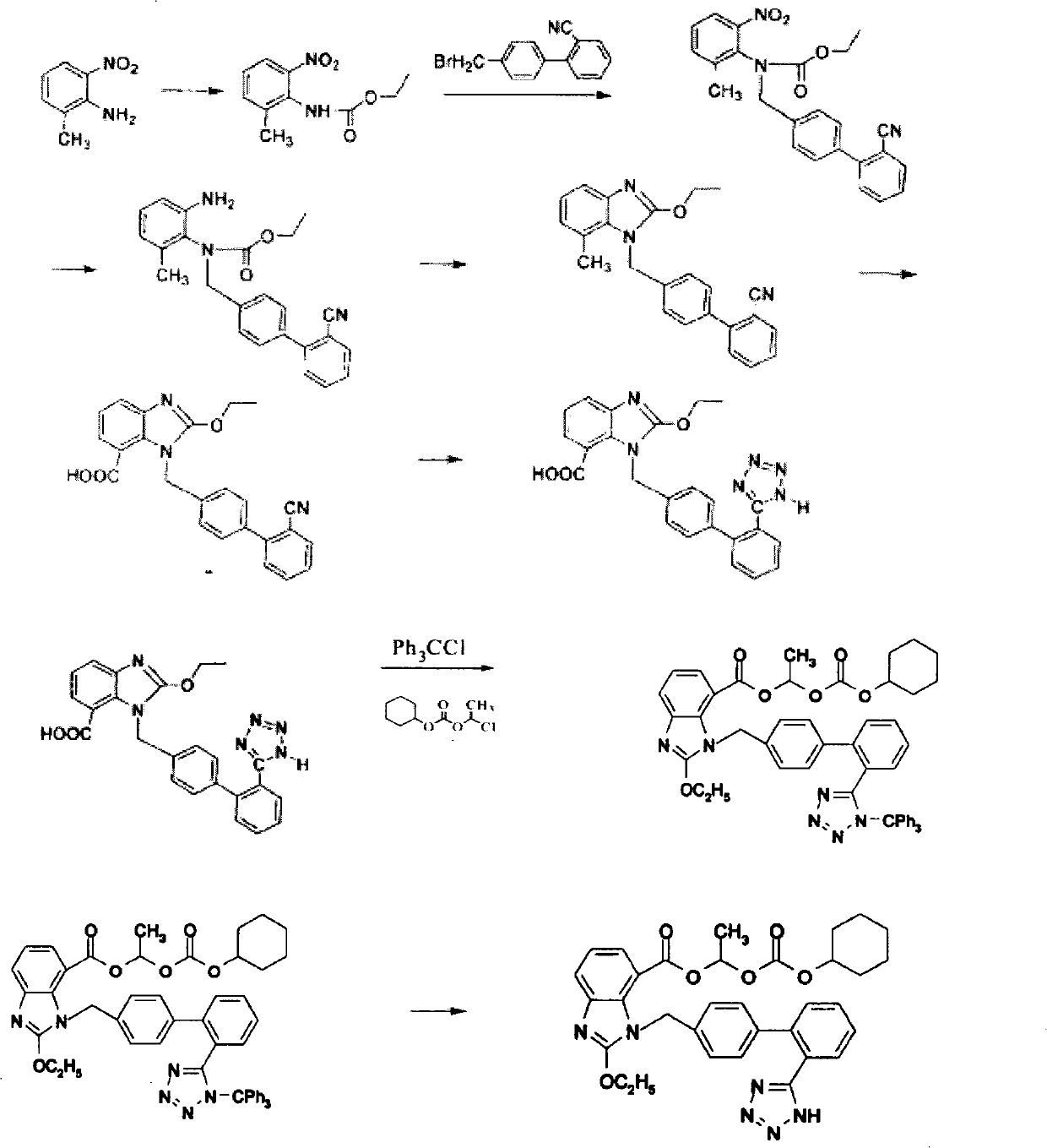
[0068] Embodiment 2 wherein: (R=ethyl, X=Br)
[0069] 1, 2-amino-3-nitrobenzoic acid;
[0070] II. Ethyl 3-nitro-2-aminobenzoate;
[0071] III, N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium;
[0072] IV, 2-N-[[(2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl]amino)- Ethyl 3-nitrobenzoate;
[0073] V, 2-N-[[(2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl]amino)- Ethyl 3-aminobenzoate;
[0074] VI, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] - ethyl 1-H-benzimidazole-7-carboxylate;
[0075] VII, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] -1-H-benzimidazole-7-carboxylic acid;
[0076] VIII, 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4-yl]methyl] -1-(cyclohexyloxycarbonyloxy)ethyl 1-H-benzimidazole-7-carboxylate;
[0077] (1), the synthesis of ethyl 3-nitro-2-aminobenzoate (II)
[0078] Add 18.2g (0.1mol) of 3-nitro-2-aminobenzoic acid into the reaction flask, suspe...
Embodiment 3
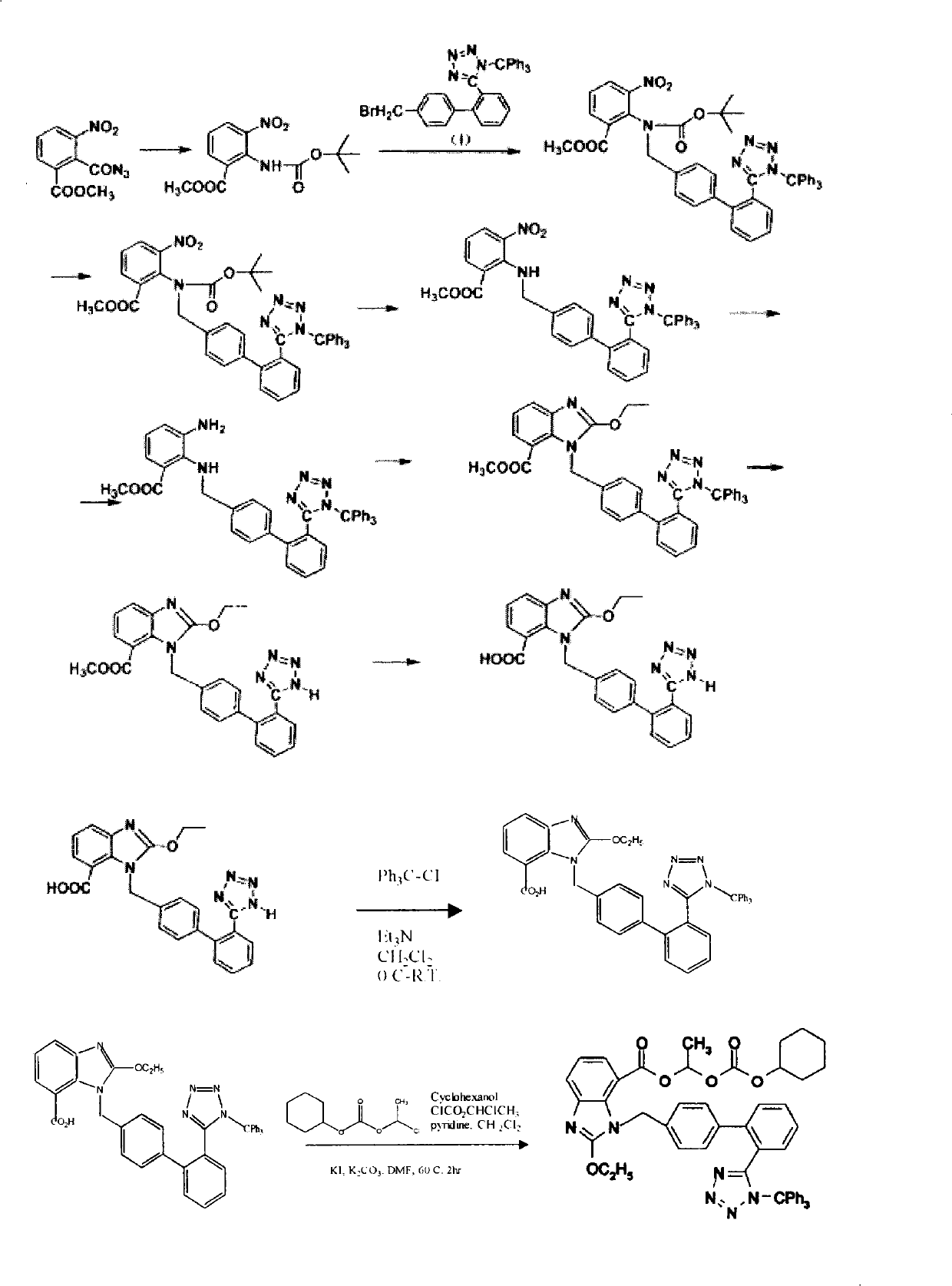
[0093] The intermediate 2-ethoxy-1-[[2'-(N'-trityl)-tetrazol-5-yl](1,1'-biphenyl)-4- Base]methyl]-1-H-benzimidazole-7-carboxylic acid 1-(cyclohexylcarbonyloxy)ethyl ester (VIII) can be obtained according to the above-mentioned Example 1 or Example 2.
[0094] Preparation of candesartan cilexetil
[0095] Add 60ml of 1N hydrochloric acid, 240ml of methanol and 50ml of chloroform to 50g of the compound VIII produced above, stir at room temperature for 3 hours, concentrate in vacuo at room temperature, extract with dichloromethane, wash with water, dry over anhydrous magnesium sulfate, concentrate in vacuo to dryness, and wash with 300ml of methanol 23.89 g of candesartan cilexetil was obtained by recrystallization, with a yield of 68% and a purity of 91%.
[0096] The average total yield of candesartan cilexetil obtained by the method is 37.78%.
[0097] Of course, in the above embodiment, Br in compound III can also be replaced by other elements in halogen, such as Cl, I, etc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com