Method for synthesizing candesartan cilexetil intermediate
A technology of nitrobenzoate and microchannel reactor, which is applied in chemical instruments and methods, preparation of organic compounds, chemical/physical/physicochemical processes, etc., and can solve high reaction heat, long production time, and raw material consumption and other problems, to achieve the effect of simple preparation process, stable product quality and short residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
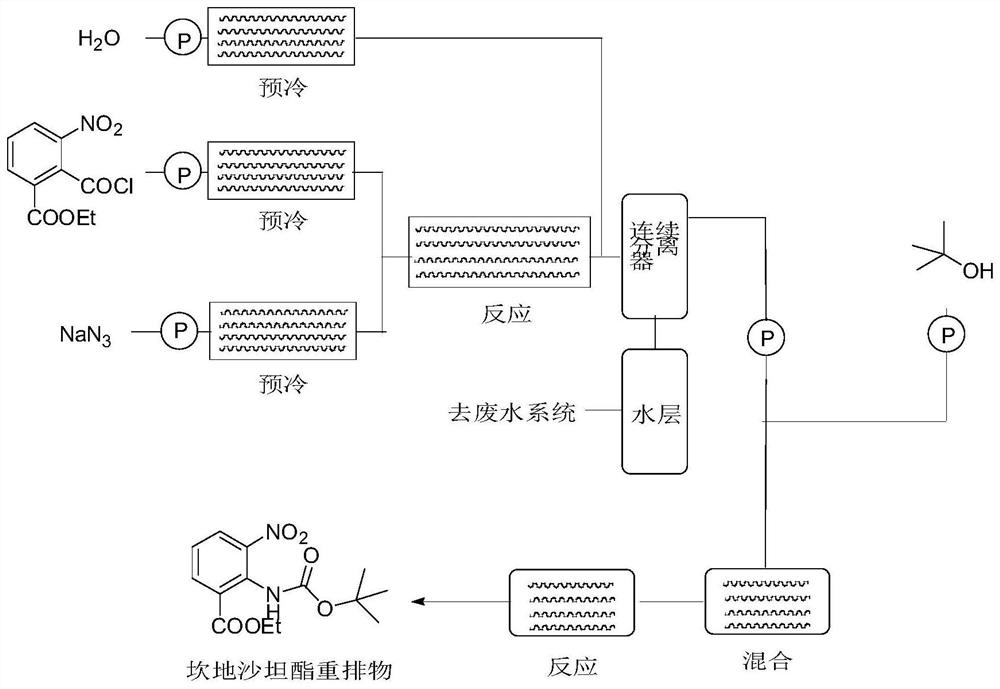
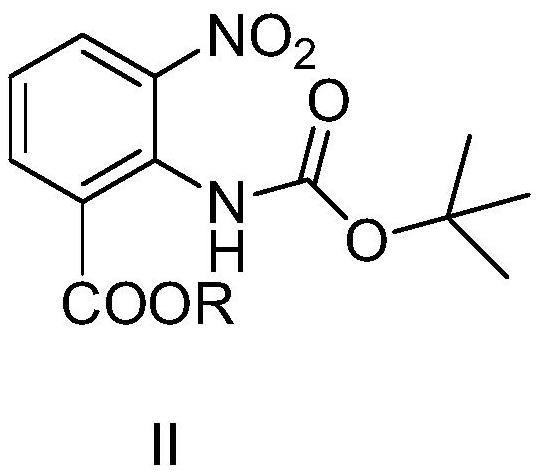
[0053] Synthesis of ethyl 2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
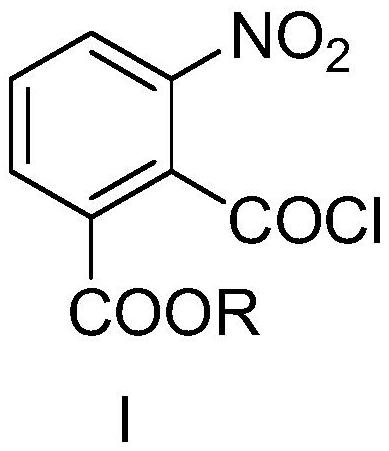
[0054]Dissolve ethyl 2-(chlorocarbonyl)-3-nitrobenzoate in a non-polar organic solvent to make a 1mol / L solution, dissolve sodium azide in a polar solvent to make a 0.5mol / L solution; In the continuous flow reactor, the ethyl 2-(chlorocarbonyl)-3-nitrobenzoate solution is passed through the first pre-cooling module with a metering pump (flow rate 1mL / s), and the temperature of the pre-cooling medium is controlled at 0°C; The sodium azide solution passes through the second pre-cooling module with a metering pump (flow rate 2mL / s), and the pre-cooling medium is controlled at 0°C; after the two solutions are mixed, they pass through the first reaction module at 0°C within 30 seconds; for drinking water The metering pump (flow rate 1mL / s) passes through the third pre-cooling module, and the pre-cooling medium is controlled at 2°C. After the feed liquid is output from the first reaction module, it is mixed w...
Embodiment 2
[0058] Synthesis of ethyl 2-((tert-butoxycarbonyl)amino)-3-nitrobenzoate
[0059] Dissolve ethyl 2-(chlorocarbonyl)-3-nitrobenzoate in toluene to make a toluene solution, dissolve sodium azide in water to make an aqueous solution; in a continuous flow reactor, 2-(chlorocarbonyl)- The toluene solution of ethyl 3-nitrobenzoate passes through the first pre-cooling module at a flow rate of 1mL / s with a metering pump, and the temperature of the pre-cooling medium is controlled at 0°C; The flow rate passes through the second pre-cooling module, and the pre-cooling medium is controlled at 0°C; after the two solutions are mixed, they pass through the first reaction module at 0°C within 30 seconds; the drinking water passes through the first reaction module at a flow rate of 0.5mL / s Three pre-cooling modules, the pre-cooling medium is controlled at 2°C, the feed liquid is output from the first reaction module, mixed with the drinking water from the pre-cooling module, and enters the co...
Embodiment 3
[0063] Synthesis of Methyl 2-((tert-Butoxycarbonyl)amino)-3-nitrobenzoate
[0064] Dissolve methyl 2-(chlorocarbonyl)-3-nitrobenzoate in toluene to make a 0.5mol / L solution, dissolve sodium azide in water to make a 0.5mol / L solution; in a continuous flow reactor , the ethyl 2-(chlorocarbonyl)-3-nitrobenzoate solution is passed through the first pre-cooling module with a metering pump (flow rate 1mL / s), and the temperature of the pre-cooling medium is controlled at 0°C; the sodium azide solution is metered The pump (flow rate 1mL / s) passes through the second pre-cooling module, and the pre-cooling medium is controlled at 0°C; after the two solutions are mixed, they pass through the first reaction module at 0°C within 30 seconds; the metering pump for drinking water (flow rate 0.5mL / s) After the third pre-cooling module, the pre-cooling medium is controlled at 2°C. After the feed liquid is output from the first reaction module, it is mixed with the drinking water from the pre-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com