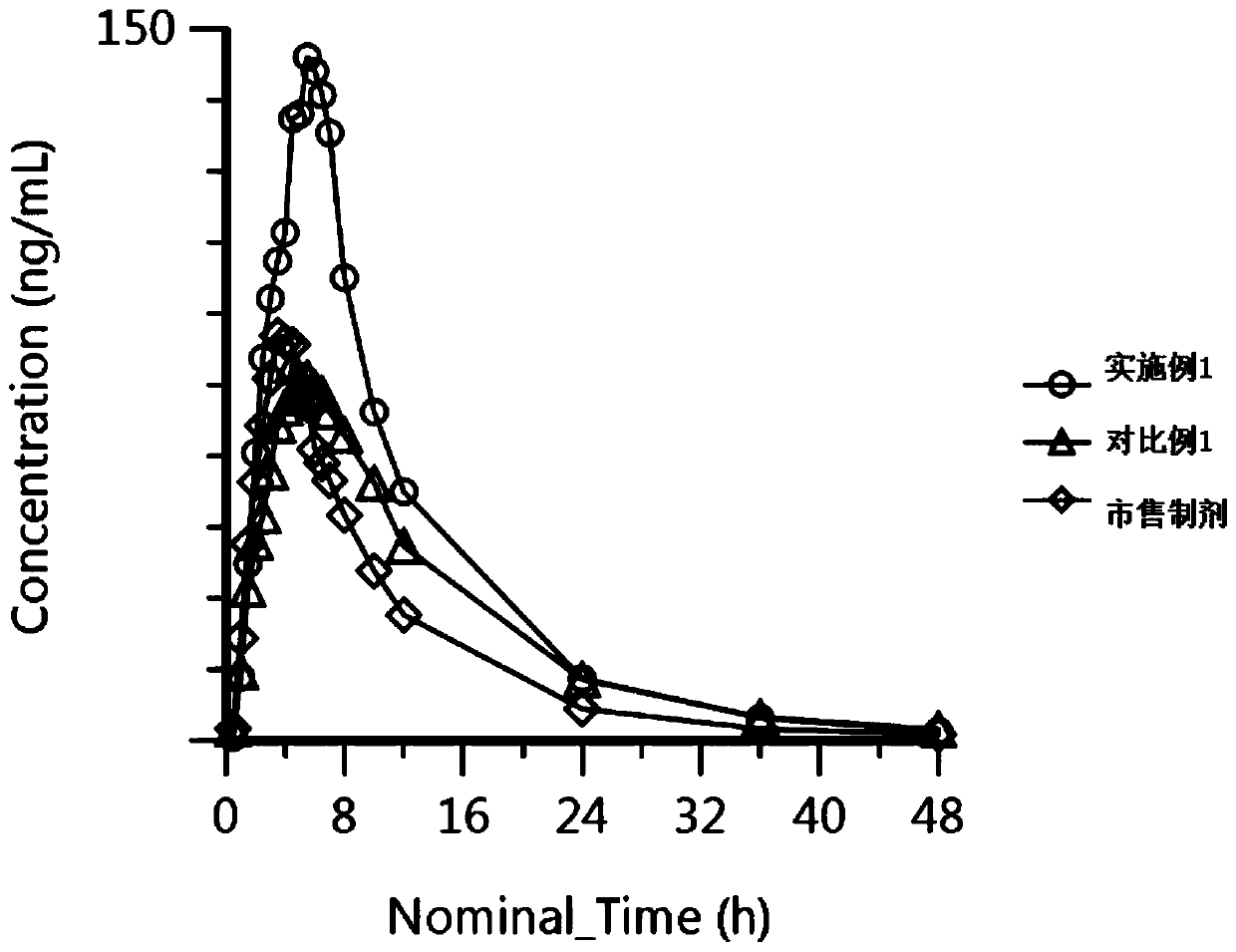
Capsule preparation containing candesartan cilexetil and preparation method of capsule preparation
A technology of candesartan cilexetil and capsules, which is applied in the field of capsule preparations containing candesartan cilexetil and its preparation, and can solve the problems of inability to improve bioavailability, low oral bioavailability, slow absorption and onset time, etc. , to achieve the effect of improving bioavailability, high dissolution, and good reproducibility of production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
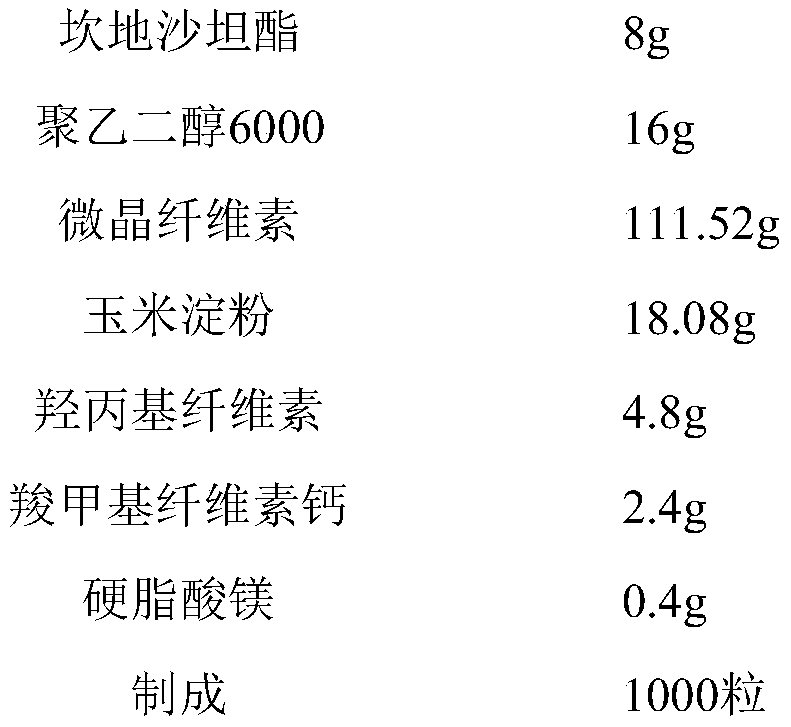
[0035] Candesartan cilexetil capsules (batch of 1000 capsules)
[0036]
[0037] Usage and dosage: once a day, 4~8mg each time.
[0038] Preparation:
[0039] a: Add candesartan cilexetil, microcrystalline cellulose, corn starch, hydroxypropyl cellulose, carmellose calcium, and polyethylene glycol 6000 into a wet granulator and stir to make the mixture uniform;
[0040] b: adding purified water to the mixture in step a to make a soft material;
[0041] c: Prepare pellets by extrusion spheronization, dry the pellets in a fluidized bed, and measure the water content below 4%;
[0042] d: adding magnesium stearate to the pellets and mixing evenly;
[0043] e; filling the mixture obtained in step d into the No. 3 gelatin capsule shell to prepare capsules containing candesartan cilexetil.
[0044] The extrusion spheronization method adopts a screen with an aperture of 1200 μm, the rotation speed of the spheronizer in the extrusion spheronization method is 1000 rpm, and the s...
Embodiment 2
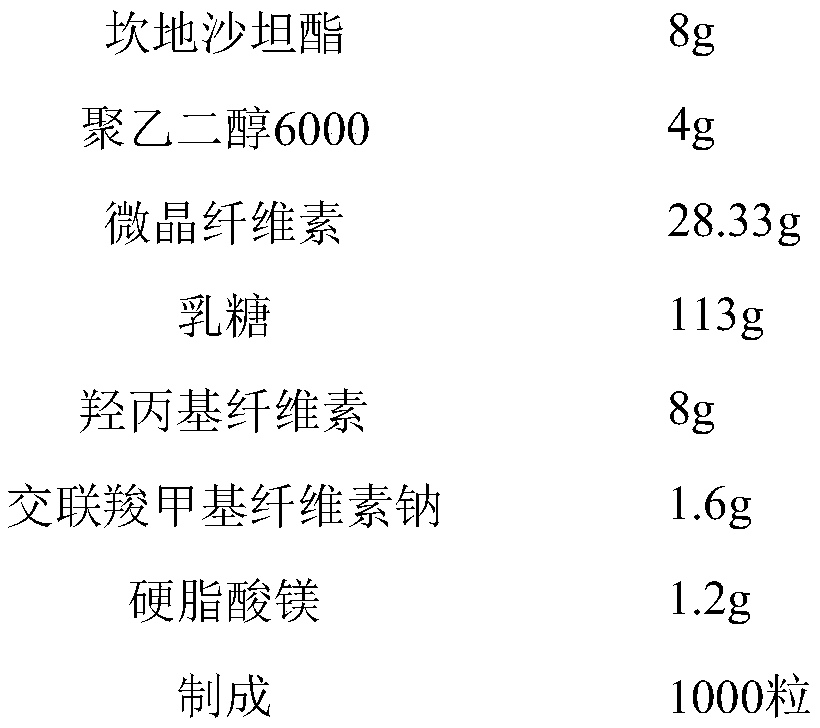
[0046] Candesartan cilexetil capsules (batch of 1000 capsules)
[0047]
[0048] Preparation:
[0049] a: Add candesartan cilexetil, microcrystalline cellulose, lactose, hydroxypropyl cellulose, croscarmellose sodium, and polyethylene glycol into a wet granulator and stir to make 6000 mix evenly;
[0050] b: adding purified water to the mixture in step a to make a soft material;
[0051] c: Prepare pellets by extrusion spheronization, dry the pellets in a fluidized bed, and measure the water content below 4%;
[0052] d: adding magnesium stearate to the pellets and mixing evenly;
[0053] e; filling the mixture obtained in step d into the No. 3 gelatin capsule shell to prepare capsules containing candesartan cilexetil.
[0054] The binder solution is obtained by adding hydroxypropyl cellulose to water and stirring evenly;
[0055] The extrusion spheronization method adopts a screen with an aperture of 600 μm, and the rotation speed of the spheronizer in the extrusion sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com