Method for efficient recombinant expression of restriction endonuclease
A restriction endonuclease and high-efficiency technology, applied in the field of bioengineering, can solve the problems of low yield of restriction endonuclease, narrow application range, cumbersome preparation procedures, etc., achieve high application and promotion value, and simplify the recombinant expression process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, a method for highly efficient recombinant expression of restriction endonucleases, comprising the following steps:
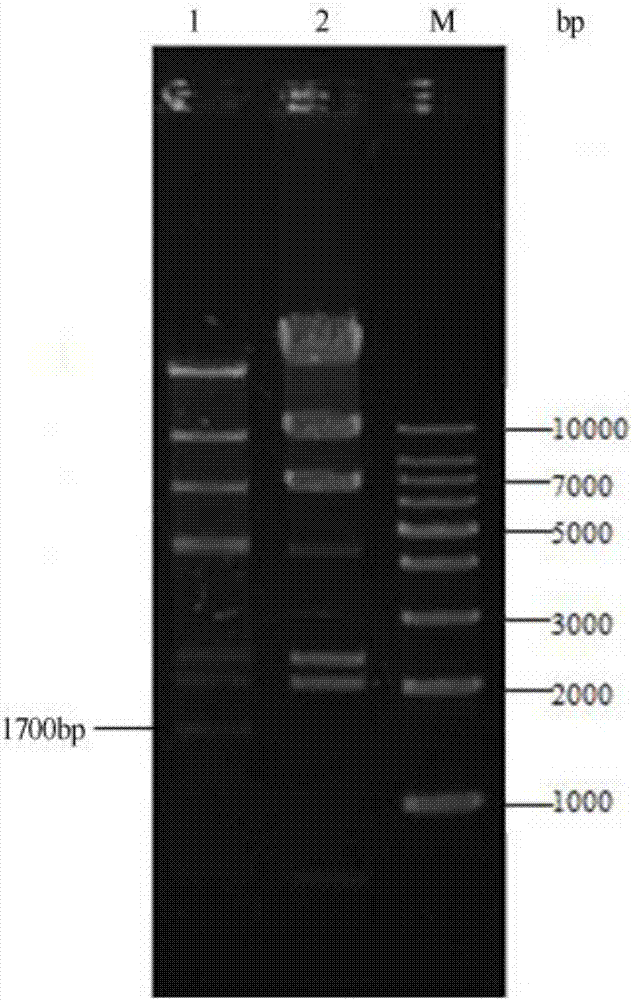
[0027] (1) Insert the restriction endonuclease gene first, then transform the vector into Escherichia coli, screen and culture, sequence, and obtain the recombinant expression plasmid of the restriction endonuclease;
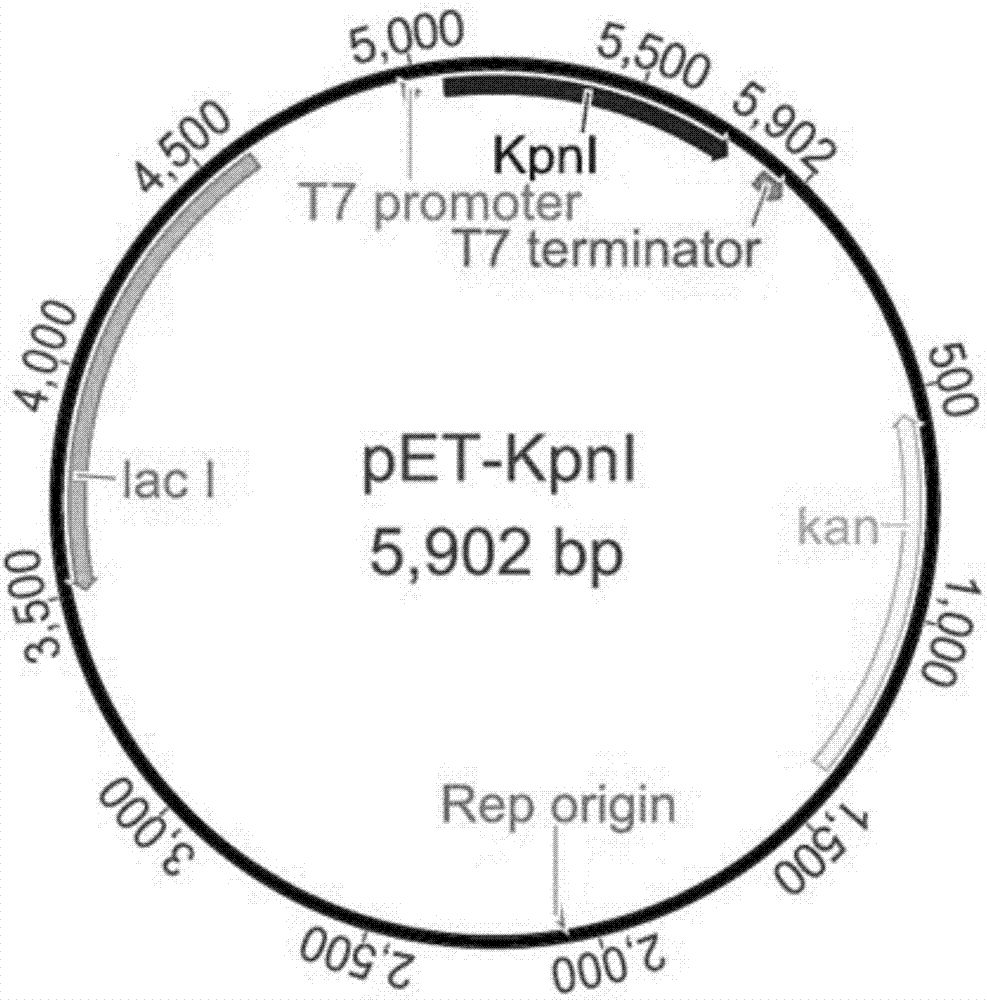
[0028]The plasmid vector is a pET series expression vector induced by IPTG under the regulation of lactose operon and T7 promoter;
[0029] (2) Transform the recombinant expression plasmid of the restriction endonuclease into BL21(DE3)pLysS competent cells, and screen to obtain the recombinant expression strain of the restriction endonuclease;
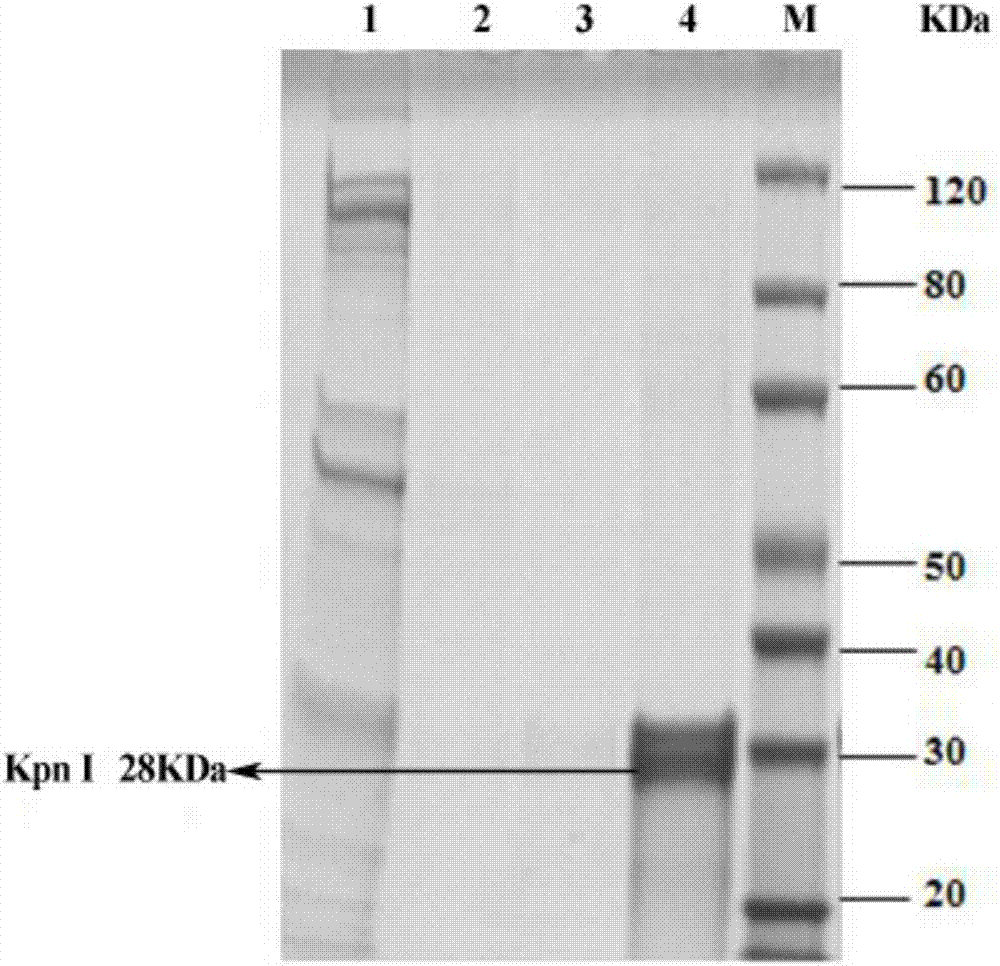
[0030] (3) Expand and cultivate recombinant expression strains of restriction endonucleases and induce the expression of restriction endonucleases.
Embodiment 2
[0031] Embodiment 2, the method for highly efficient recombinant expression of restriction endonuclease described in embodiment 1, described restriction endonuclease is selected from: BclI, DpnI, DpnII, EcoRI, HindIII, KpnI, MluI, MseI, NcoI, NdeI, NheI, NotI, NsiI, NspV, PstI, SalI, SbfI, SgeI, SspI, StuI, TaqI, XbaI, XhoI.
Embodiment 3
[0032] Example 3, the method for highly efficient recombinant expression of restriction endonucleases described in Example 1, the plasmid vector is pET-28b.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com