Compound, muscarinic m receptor antagonist, composition and application
A receptor antagonist and compound technology, which is applied in the discovery and application field of the action mechanism of the active ingredients of natural medicines, to achieve the effect of broadening the scope of clinical application, small side effects, and good effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
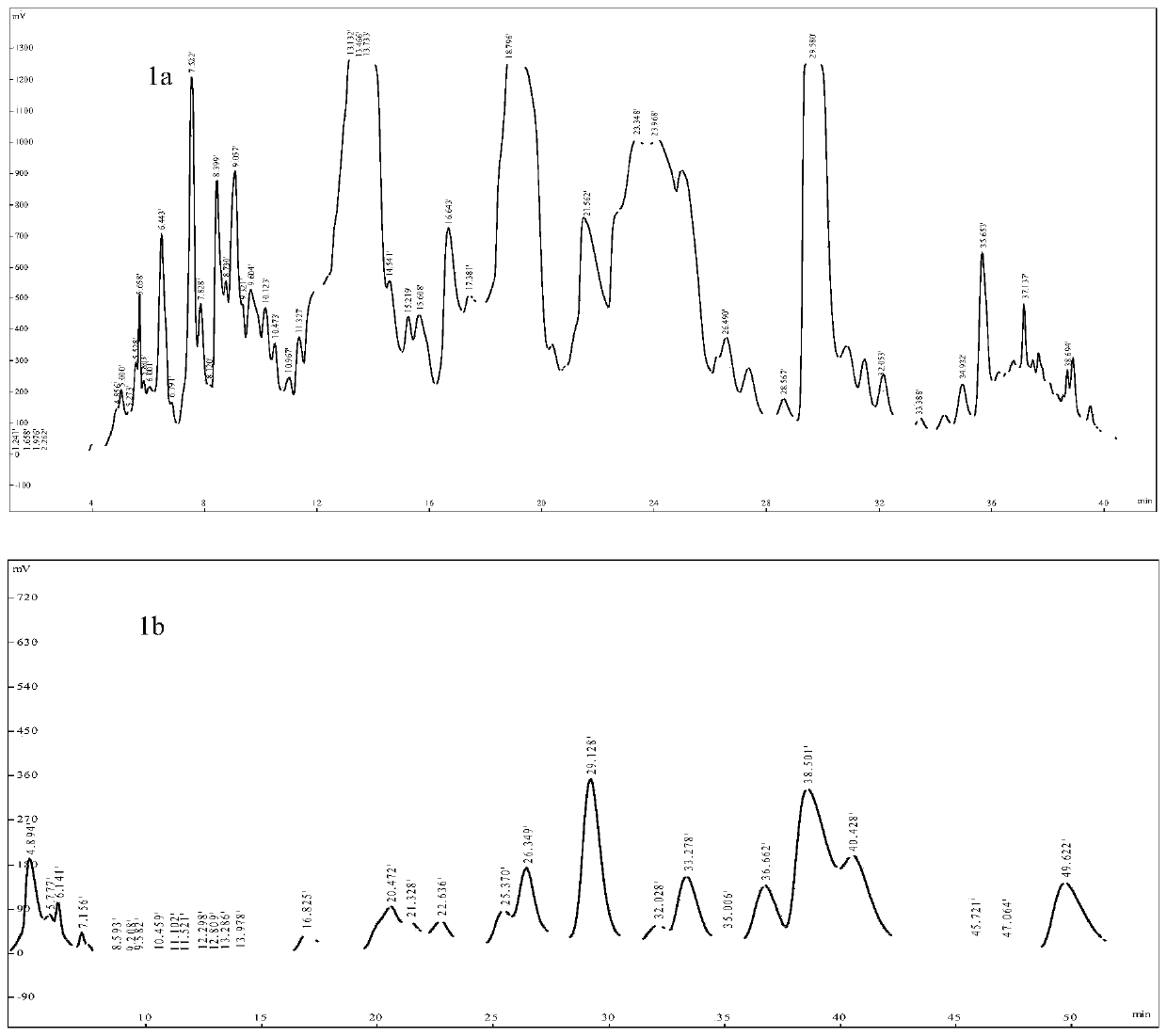
[0038] Example 1 Purification and Preparation of Alkaloid Compounds in Anisodamine Tangut
[0039] 1. Experimental instruments and reagents
[0040] Tangut Anisopolamine medicinal material was collected in Qinghai by the Northwest Plateau Botanical Institute, and was identified by Teacher Mei Lijuan. Column XCharge C18 (4.6×250mm, 7μm, Huapu Company), XCharge C18 (100×280mm, 7μm, Huapu Company), XCharge SCX (100×316mm, 7μm, Huapu Company), XCharge C18 (20×250mm , 7 μm, Huapu Company), anhydrous sodium sulfate, sodium dihydrogen phosphate dihydrate were purchased from Sinopharm Group, ethanolamine, phosphoric acid, and formic acid were purchased from Bailingwei, and preparative chromatography grade acetonitrile was purchased from Anhui Shilian.
[0041] 2. Purification and preparation of compounds
[0042] Extraction is divided into two batches, each batch is 25kg, soaked in 80L ethanol, heated to reflux for 2h, and extracted three times respectively to obtain 350L extract. ...
Embodiment 2
[0043] The DMR signal characteristic of embodiment 2 muscarinic M receptor antagonist on Epic platform
[0044] 1. Method
[0045] 1.1 Cell culture
[0046] HT29 human colon cancer cells were obtained from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences (Shanghai, China). HT29 cells were treated with McCoy’s 5A medium (GIBCO, product number 12800017, containing D-Glucose 4500.0mg / L, adding NaHCO 3 2.2g / L), at 37°C, with a volume content of 5% CO 2 (air) incubator.
[0047] 1.2 Cell Viability Experimental Method of Muscarinic M Receptor Antagonists
[0048] HT29 cells were treated with 2×10 4 Individuals / well density seeded to In a 384-well biosensor microplate, placed at 37°C, with a volume content of 5% CO 2 (air) incubator for 22 h, washed with HBSS buffer once before detection, then added 30 μL of HBSS buffer to each well, placed in The system was equilibrated and incubated for 1h. After balancing first in Establish a ...
Embodiment 3
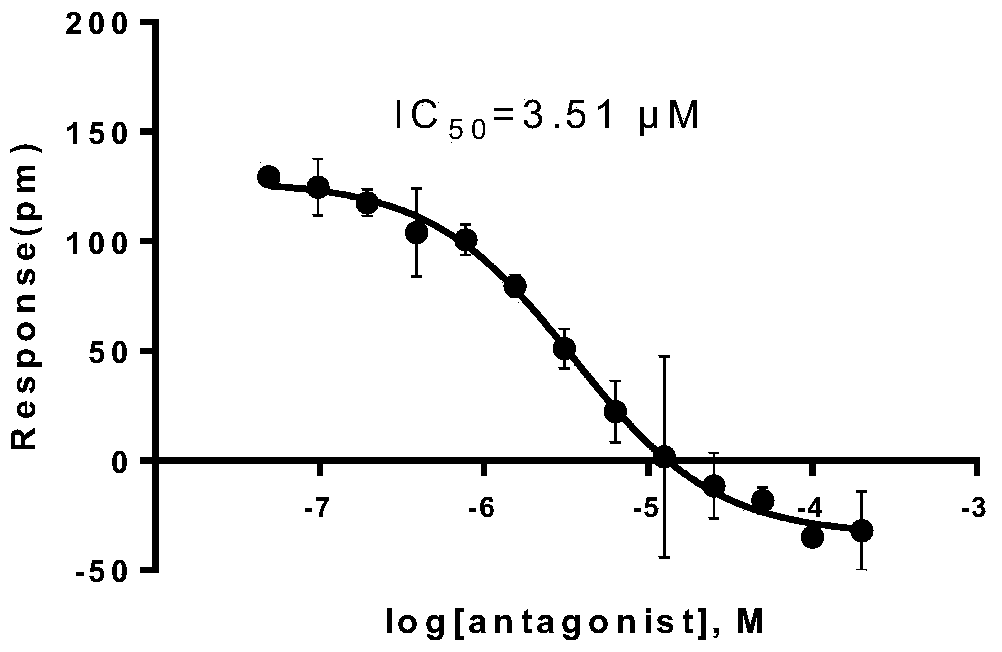
[0053] Example 3 MMPVP has antagonistic activity on muscarinic M receptors
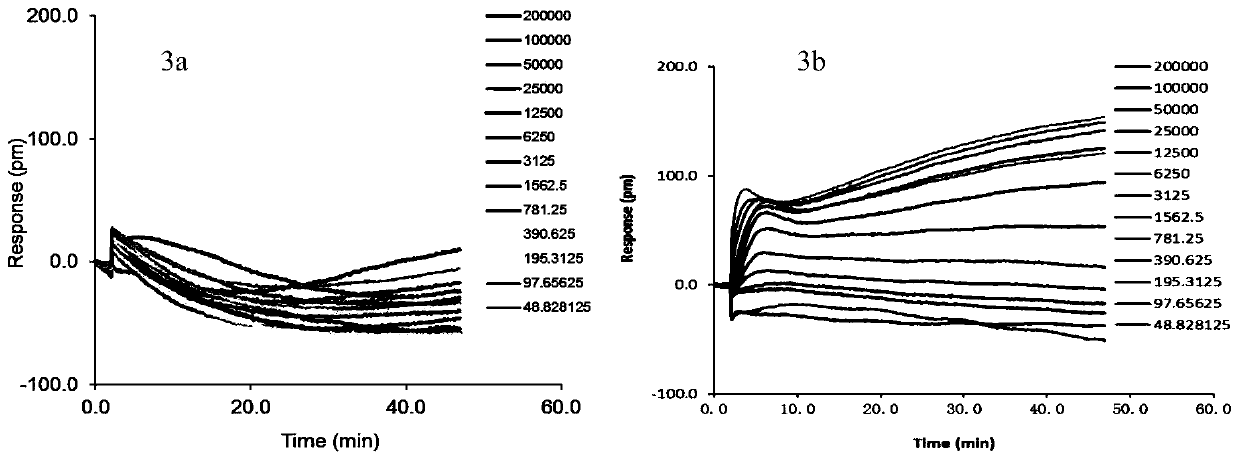
[0054] The dose-effect relationship of MMPVP, a compound with strong M receptor antagonistic activity in activity screening, was investigated using label-free cell target pharmacology techniques. First, 10 μL of the compound to be tested was added to the inoculated HT29 cells 384-well biosensor microplates, in The system was monitored for 60 minutes, and then 10 μL of acetylcholine (16 μM) was added to continue monitoring for 60 minutes. The DMR signal induced by the compound is plotted with the action time, such as image 3 a (the compound concentration is diluted stepwise from 200 μM to 48.8nM, and the corresponding DMR curve has little change); the DMR response signal caused by adding acetylcholine in the second step is plotted with the action time, and the results are as follows image 3 b (The compound concentration was serially diluted from 200 μM to 48.8 nM, and the corresponding DMR signa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com