A method for synthesizing 1,4-dihydropyridine derivatives
A technology of dihydropyridines and derivatives, applied in organic chemistry, chemical recovery, etc., can solve the problems of rapid detection of catalyst residues, long time, difficult separation, etc., achieves significant industrialization prospects, avoids pollution, and has a wide range of raw material sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
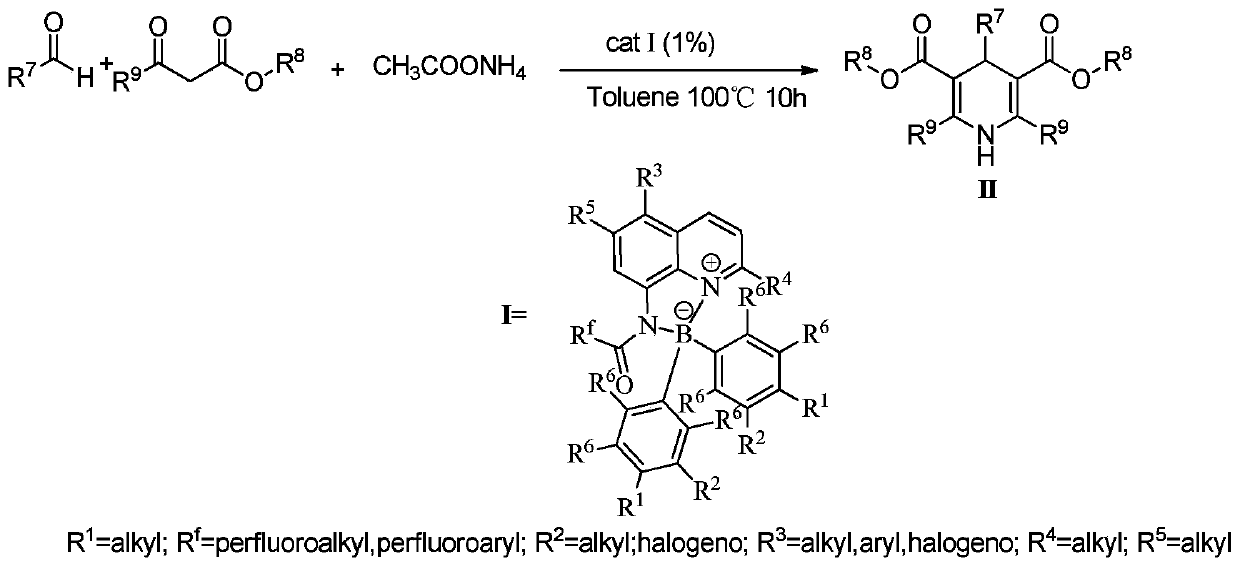
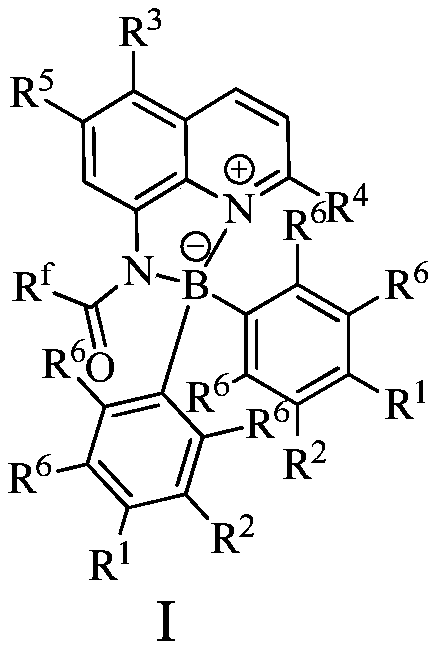
[0023] In a 100mL single-necked flask, add 0.01mol% Lewis acid-base bifunctional catalyst I (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.1mol benzaldehyde (R 7 =Ph), 0.1mol methyl acetoacetate (R 8 = Me; R 9 =Me), 0.1mol ammonium acetate, 10mL toluene, the reaction was stirred at 100°C for 10 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = Ph; R 8 = Me; R 9 =Me) yield was 87%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
preparation example 2
[0025] In a 100mL single-necked flask, add 0.03mol% Lewis acid-base bifunctional catalyst I (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.1mol benzaldehyde (R 7 =Ph), 0.1mol methyl acetoacetate (R 8 = Me; R 9 =Me), 0.1mol ammonium acetate, 10mL toluene, the reaction was stirred at 100°C for 10 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = Ph; R 8 = Me; R 9 =Me) yield was 88%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
preparation example 3
[0027] Add 0.08mol% Lewis acid-base bifunctional catalyst I (where R f = CF 3 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 =H), 0.1mol benzaldehyde (R 7 =Ph), 0.1mol methyl acetoacetate (R 8 = Me; R 9 =Me), 0.1mol ammonium acetate, 10mL toluene, the reaction was stirred at 100°C for 10 hours, followed by TLC until the reaction was complete. The reaction result is: product II (R 7 = Ph; R 8 = Me; R 9 =Me) yield was 88%; after the catalyst system was reused 10 times, its catalytic performance did not decrease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com