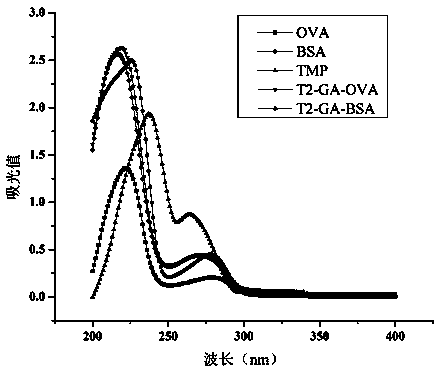
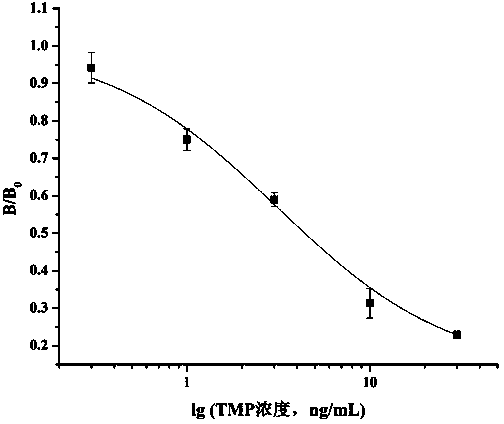
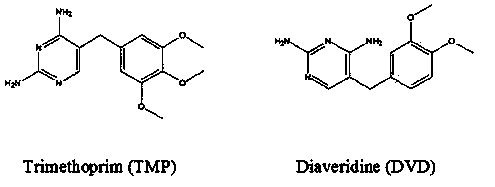
Method for preparing complete antigen from trimethoprim hapten T2 and application of complete antigen
A technology of trimethoprim and complete antigen, which is applied in the field of preparing complete antigen from trimethoprim hapten T2, and achieves the effects of high specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Trimethoprim Hapten T2
[0031] Add 10mL of pyridine, 3.2g of p-aminobenzoic acid, and 5mg of 4-dimethylaminopyridine in sequence to a 100mL three-necked flask, and heat in a water bath at 60°C with magnetic stirring until the solution is clear. Dissolve 2.9g of trimethoprim in 10mL of pyridine and add it dropwise into a three-neck flask, continue to stir for 3 days, and use thin-layer chromatography with a volume ratio of methanol: tetrahydrofuran: chloroform of 1:1:5 as the developer Monitor the progress of the reaction. Distill at 60°C for 10 minutes under reduced pressure of 0.092 MPa to evaporate all the solvent to obtain a yellow oil. Add 10% (m / v) NaHCO dropwise 3 The solution was no longer turbid, filtered and the supernatant was recrystallized under acidic conditions of pH 5.0 to precipitate a white solid, centrifuged at 7500 r / min for 8 min, and the filter cake was dried at 60°C to obtain the trimethoprim hapten T2.
Embodiment 2
[0032] Example 2 Preparation of Trimethoprim Complete Antigen T2-ZD-BSA
[0033] Take 8 mg of trimethoprim hapten T2, dissolve it in 1 mL of DMF and mix it with 1N hydrochloric acid (the molar ratio of T2 and hydrochloric acid is 1:2.5). % sodium nitrite solution, with starch-potassium iodide test paper, it was blue-black, and the stirring reaction was continued for 2 hours to obtain the activated trimethoprim hapten T2 solution. Take BSA (the molar ratio of hapten T2 to BSA is 50:1 and 100:1, respectively), and dissolve it in 0.1M pH9.6 carbonate buffer solution, wherein the dissolved protein concentration is greater than 3mg / mL, and the carbonate The ratio of buffer to DMF was 5:1. Slowly add 14.5 mg / mL activated trimethoprim hapten T2 solution dropwise to 22 mg / mL BSA solution 3 mL, react at room temperature for 12 h, dialyze with PBS buffer for 3 days, change the water 8 times during the period, and obtain 50 ︰1 and 100︰1 two complete trimethoprim antigens (T2-ZD-BSA). ...
Embodiment 3
[0034]Example 3 Preparation of immunogen (T2-GA-BSA) and coating agent (T2-GA-OVA)
[0035] Take 8 mg of trimethoprim hapten T2, dissolve it in 1 mL of DMF, react with glutaraldehyde (the molar ratio of T2:glutaraldehyde is 1:1.5), stir and react for 2 h at 4 °C, and then react at room temperature at 25 °C Reaction 12h. Take BSA / OVA (the molar ratio of hapten T2 to BSA and OVA is 100:1) and dissolve it in 0.1M pH9.6 carbonate buffer, where the dissolved protein concentration is greater than 3mg / mL, and the carbonate The volume ratio of buffer to DMF is 5:1. Slowly add 14.5 mg / mL activated trimethoprim hapten T2 solution dropwise to 3 mL of protein 22 mg / mL BSA / OVA solution, react at room temperature for 24 hours, dialyze with PBS buffer for 3 days, change the water 8 times during the period, That is, trimethoprim immunogen (T2-GA-BSA) and coating agent (T2-GA-OVA) were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com