Use of methylnaltrexone to attenuate tumor progression
A methylnaltrexone, tumor technology, applied in the field of using methylnaltrexone to slow tumor progression, can solve problems such as constipation, reduction of intestinal transit rate, severe itching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
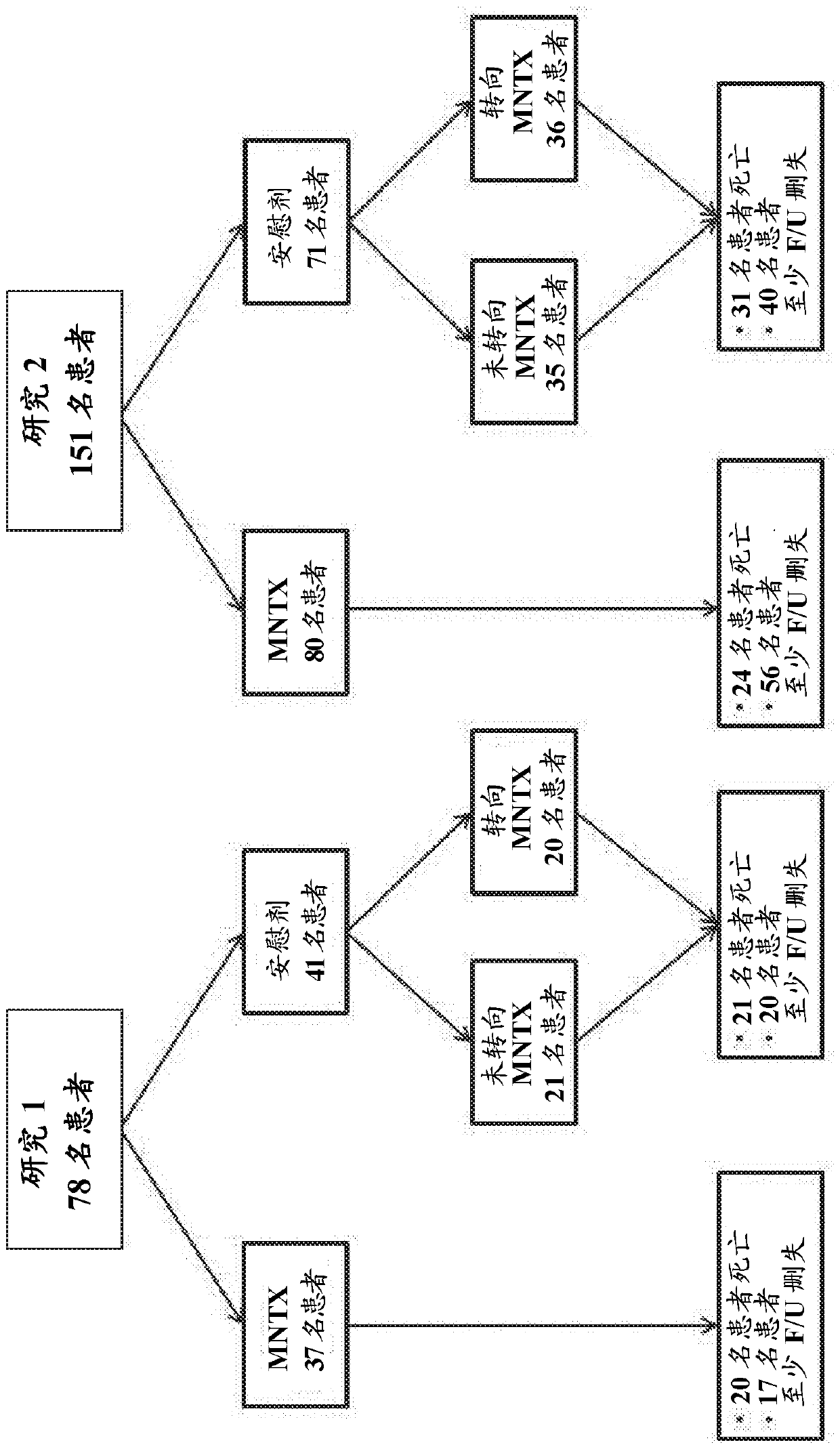
[0383] Example 1: Study 1
[0384] A double-blind randomized placebo-controlled study of subcutaneous methylnaltrexone (MNTX) was conducted in subjects with advanced medical disease and a prior history of opioid-induced constipation. To meet the eligibility criteria for recruitment, subjects were required to start a stable dose of laxative and opioid analgesics. In the context of the study, a stable dose of opioid analgesic was defined as no decrease in the dose of opioid analgesic by ≥ 50% for at least 3 days prior to the first dose of MNTX. Stable laxative dosing was defined as a long-term laxative order (not PRN or as needed) for at least 3 days prior to the first dose of MNTX. If the subject has had less than 3 bowel movements in the previous few weeks according to the medical history and has no clinically significant bowel movement within 24 hours before the first dose of MNTX, or has no clinically significant bowel movement within 48 hours before the first dose of MNTX ...
Embodiment 2
[0394] Example 2: Study 2
[0395]A double-blind randomized placebo-controlled study of subcutaneous MNTX was conducted in subjects with advanced medical disease and a prior history of opioid-induced constipation. To meet the eligibility criteria for recruitment, subjects were required to start a stable dose of laxative and opioid analgesics. In the context of the study, a stable dose of opioid analgesic was defined as no decrease in the dose of opioid analgesic by ≥ 50% for at least 3 days prior to the first dose of MNTX. Stable laxative dosing was defined as a long-term laxative order (not PRN or as needed) for at least 3 days prior to the first dose of MNTX. If the subject (a) has had less than 3 bowel movements in the previous few weeks according to the medical history and has not had a clinically significant bowel movement within 24 hours before the first dose of MNTX, or (b) 48 hours before the first dose of MNTX In the absence of clinically significant laxation, a dia...
Embodiment 3
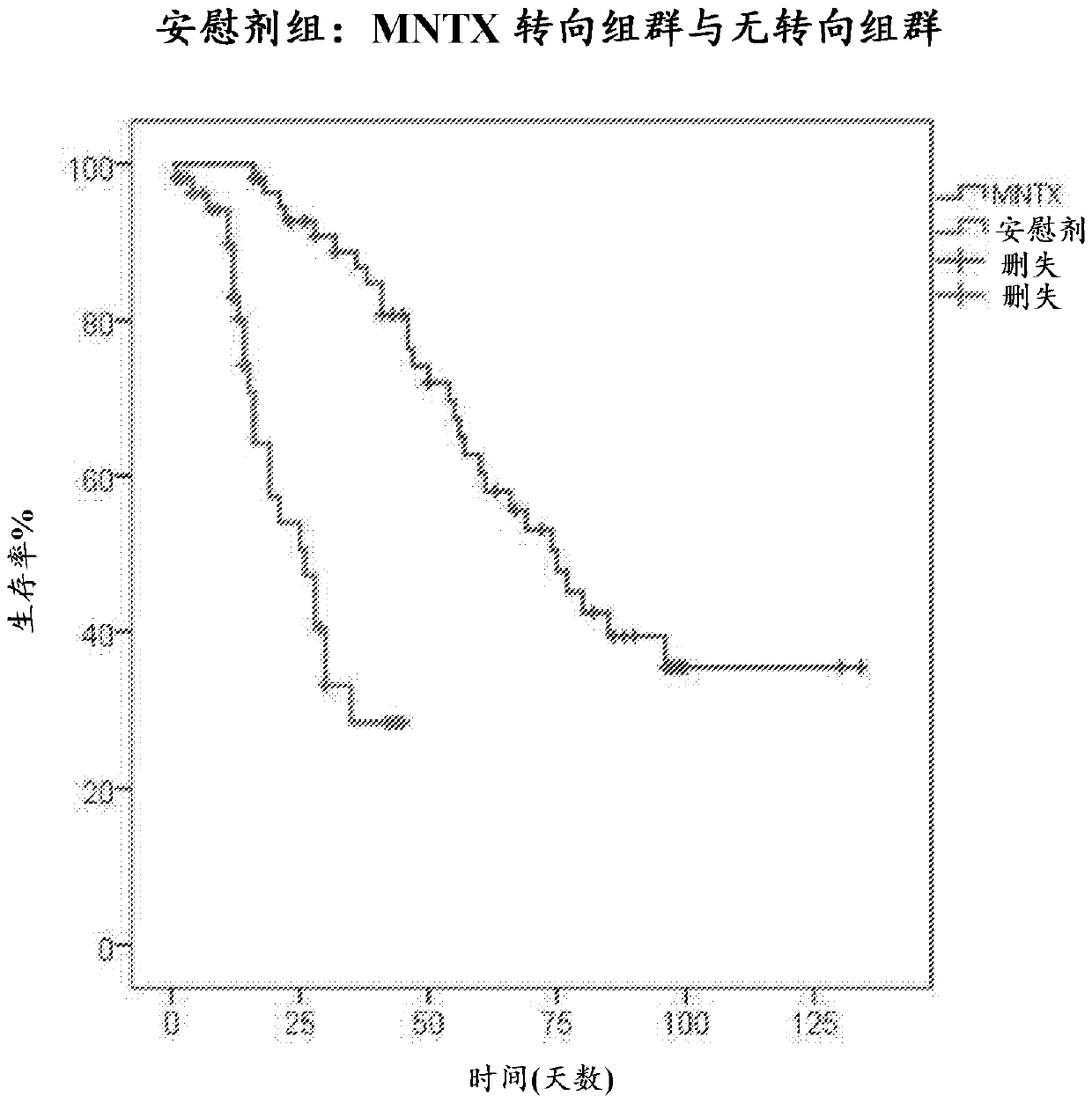
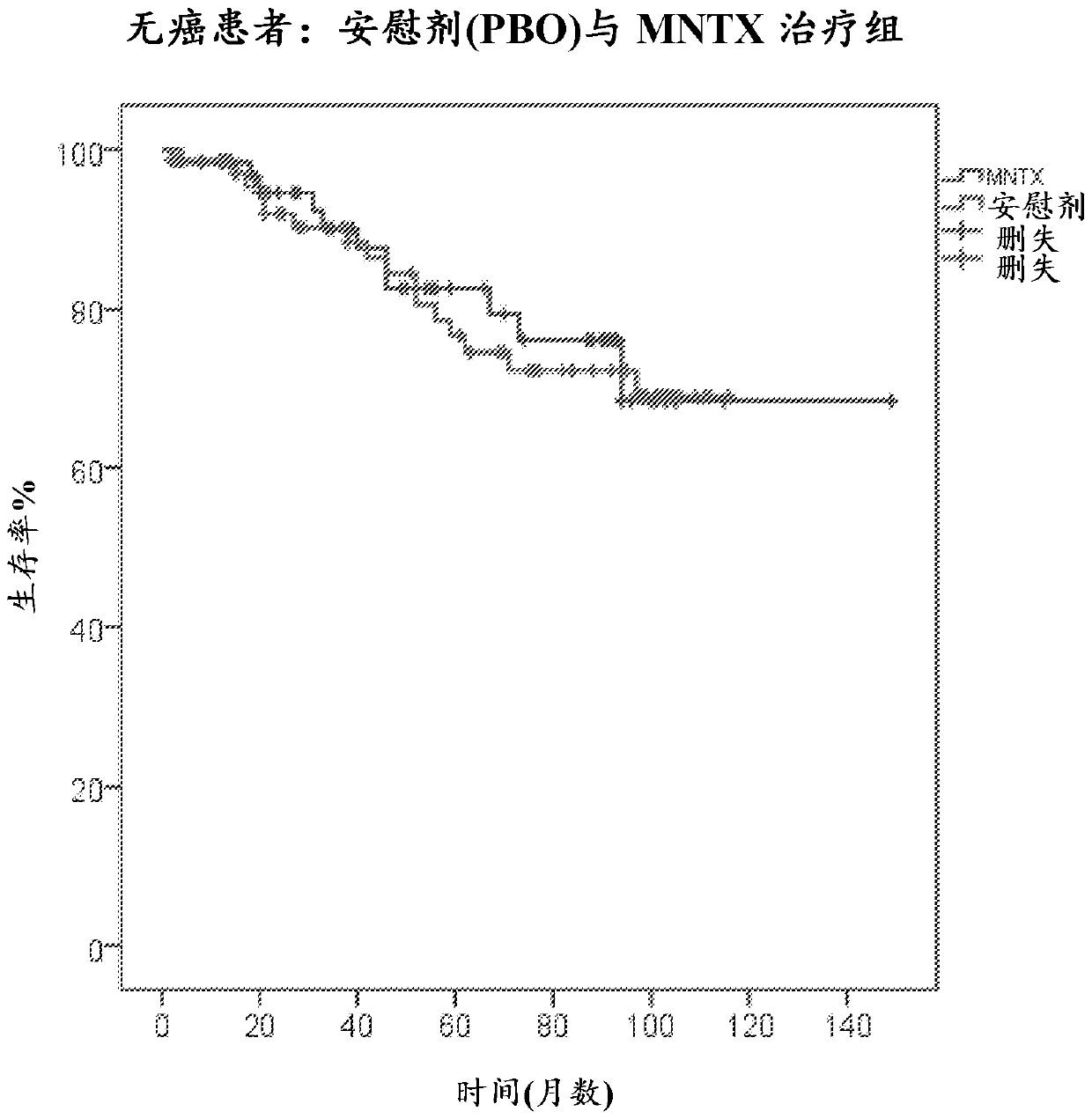
[0408] Methylnaltrexone (MNTX) is approved for the treatment of opioid-induced constipation (OIC) in subjects with advanced disease receiving palliative care when the response to laxative therapy is inadequate. Because MNTX is restricted across the blood-brain barrier, it can be administered to cancer subjects receiving opioid therapy without affecting analgesia. Recent cellular, molecular, animal and human data suggest that the mu opioid receptor (MOR) can be a target for chemotherapeutic agents. MNTX has been shown to potentially slow cancer progression. In animal models, mu opiate receptor (MOR) antagonists reduce tumor growth in lung, head and neck, breast and pancreatic tumors at clinically relevant doses. MOR knockout mice show reduced tumor growth and metastasis in lung cancer and melanoma. In a Lewis lung cancer model, infusion of MNTX significantly reduced growth and metastasis. In addition, polymorphisms of MOR that confer opioid resistance significantly prolong s...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap