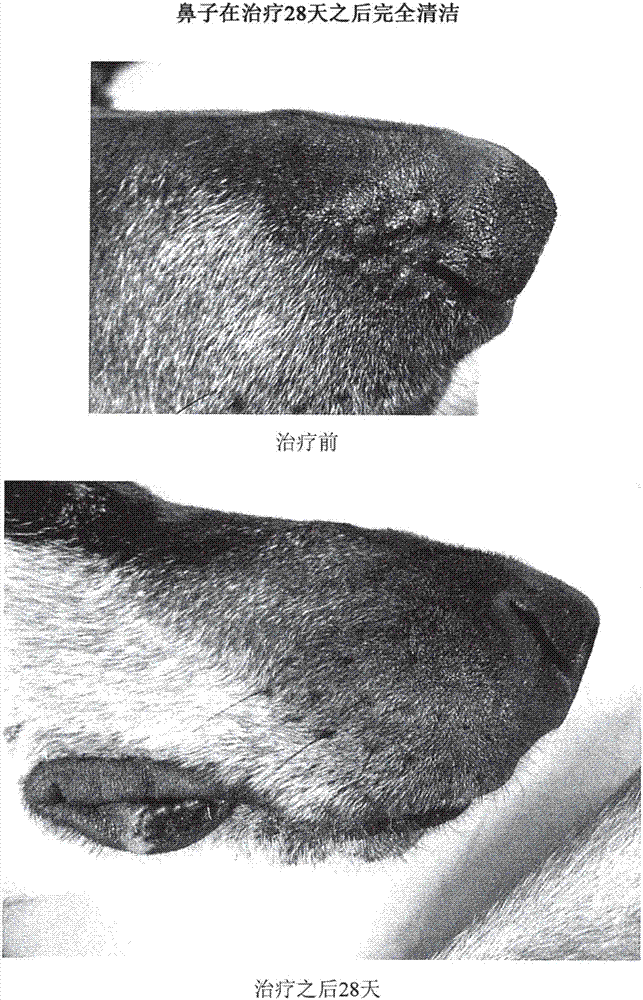
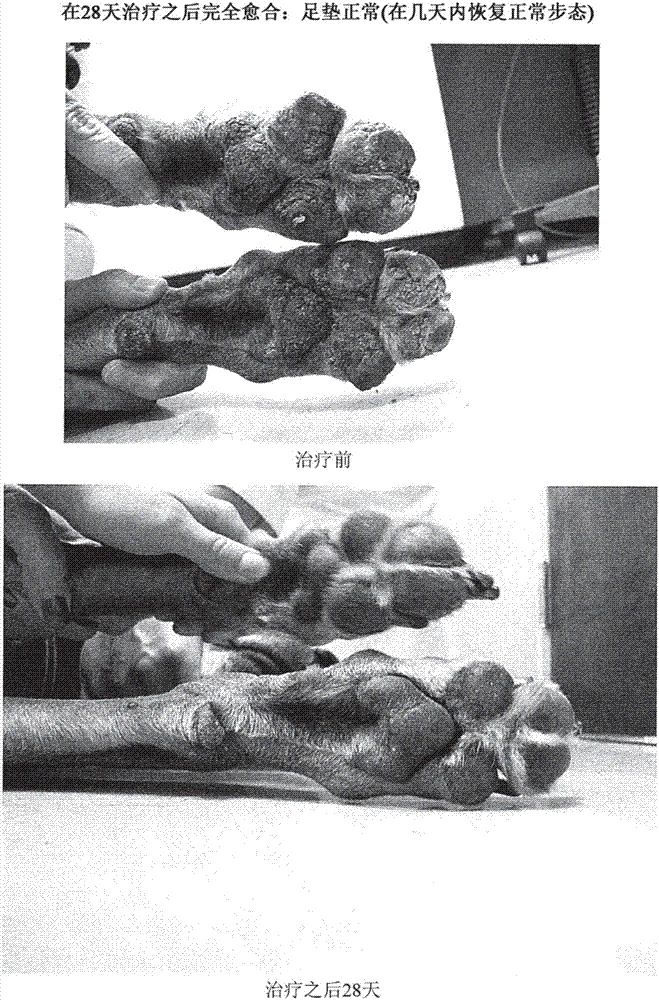
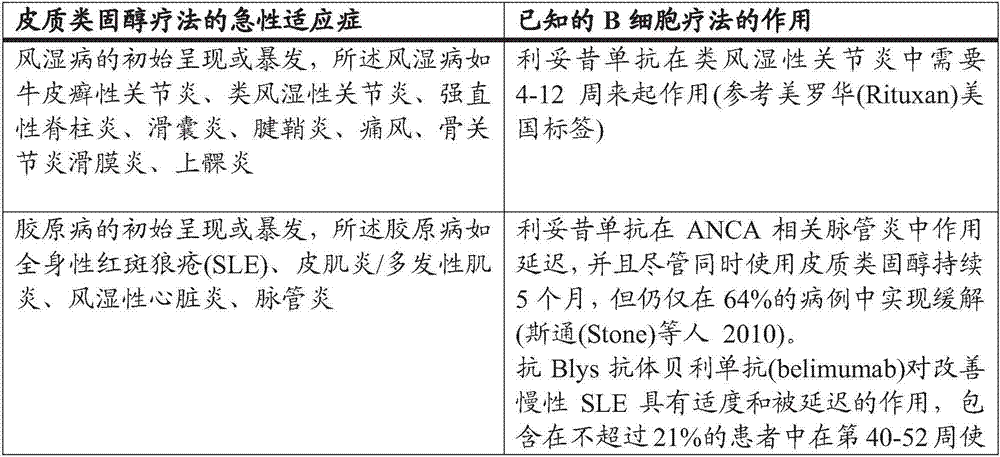
Treatment of pemphigus
A kind of therapy, corticosteroid technology, applied in medical preparations containing active ingredients, allergic diseases, peptide/protein components, etc., can solve the problems of expensive IVIG therapy, adverse effects, delayed clinical response of rituximab, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] Accordingly, in a first aspect there is provided a method of treating an autoimmune blistering disease in a mammal in need thereof, said method comprising administering to said mammal in need of said treatment a therapeutically effective amount of a BTK inhibitor .
[0013] In a second aspect, there is provided a method of treating acute inflammatory and / or autoimmune diseases in a mammal in need thereof, wherein corticosteroid therapy is used as first-line or second-line therapy, said method comprising introducing The treated mammal is administered a therapeutically effective amount of a BTK inhibitor instead of or in combination with the corticosteroid therapy; and
[0014] The BTK inhibitor is optionally administered in combination with a non-corticosteroid immunosuppressant and / or anti-inflammatory agent.
[0015] In a third aspect, there is provided a method of treating an inflammatory and / or autoimmune disease (wherein corticosteroid therapy is used as first-line...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com