Use of thymosin alpha 1 for the treatment of cystic fibrosis
A cystic fibrosis, thymosin technology, used in the digestive system, organic active ingredients, non-central pain relievers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
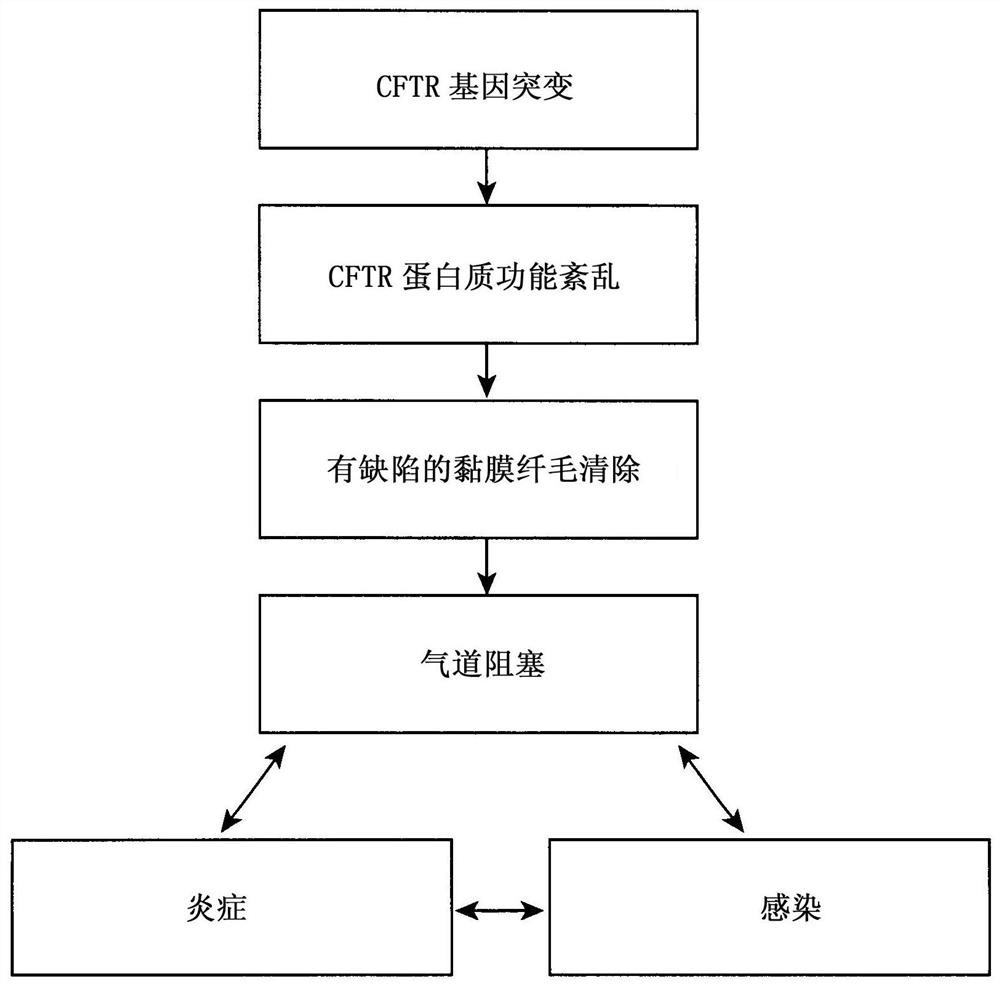
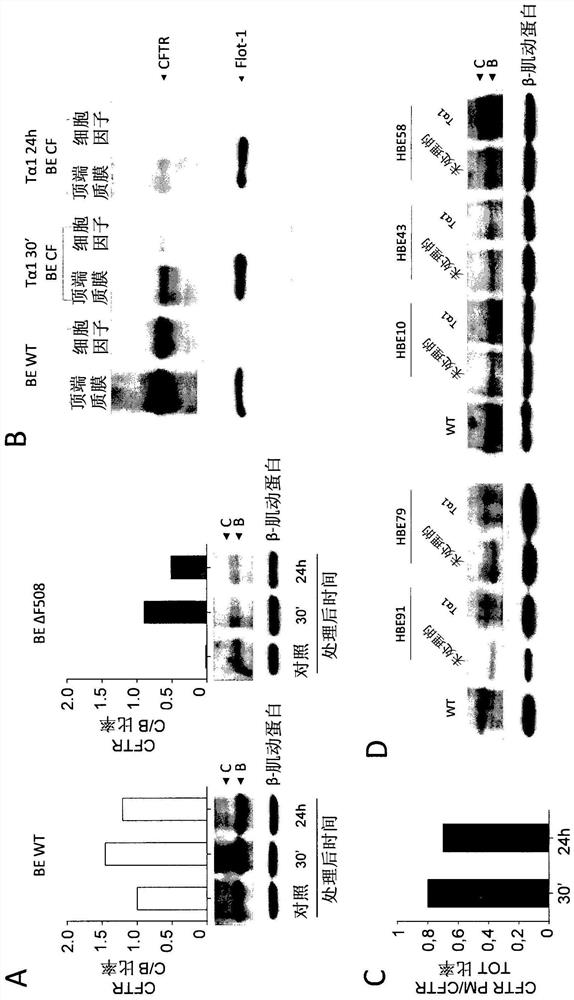
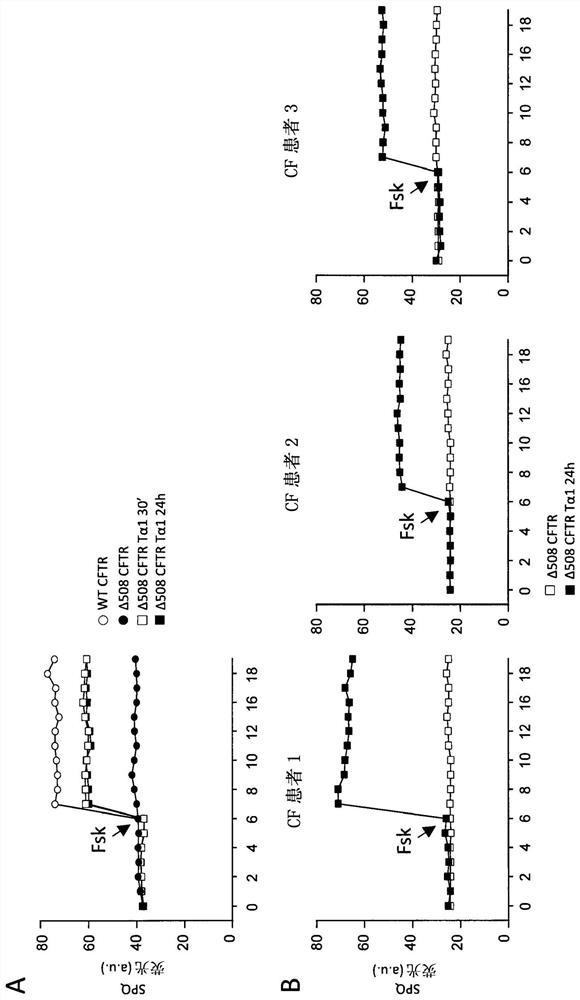
[0048] ESEMPIO 1: Studies in cystic fibrosis implicate the role of thymosin α1 as a CFTR corrector and potentiator and anti-inflammatory agent.
[0049] Materials and methods
[0050] cell. Cell Lines and Cell Cultures - Human bronchial epithelial (HBE) cells, homozygous for the deltaF508 mutation, and its isogenic wild-type obtained from lung transplantation (CF patients) or lung resection (non-CF patients) (courtesy of Cystic Fibrosis, Italy Courtesy of LJ Galietta of the Foundation). Keep cells in air with 5% CO 2In a 37°C humidified incubator, the experiment will be completed 5 days after transplantation (21, 22). Stable lentivirus-based transduction of parental CFBE41o-cells (ΔF508 / ΔF508), originally specimend and characterized by Dr. D. Gruenert and colleagues (32) with WT-CFTR or ΔF508-CFTR, was performed by Tranzyme (Birmingham, AL). The transduced CFBE41o-cells were maintained in a medium containing 50 units / ml penicillin, 50 μg / ml streptomycin, 2 mM L-glutamine,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com