Dual loaded liposomal pharmaceutical formulations
一种脂质体、药物的技术,应用在脂质体输送、药物组合、药物输送等方向,能够解决产品异质性、低可重复性等问题,达到降低毒性、减轻不期望的副作用的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
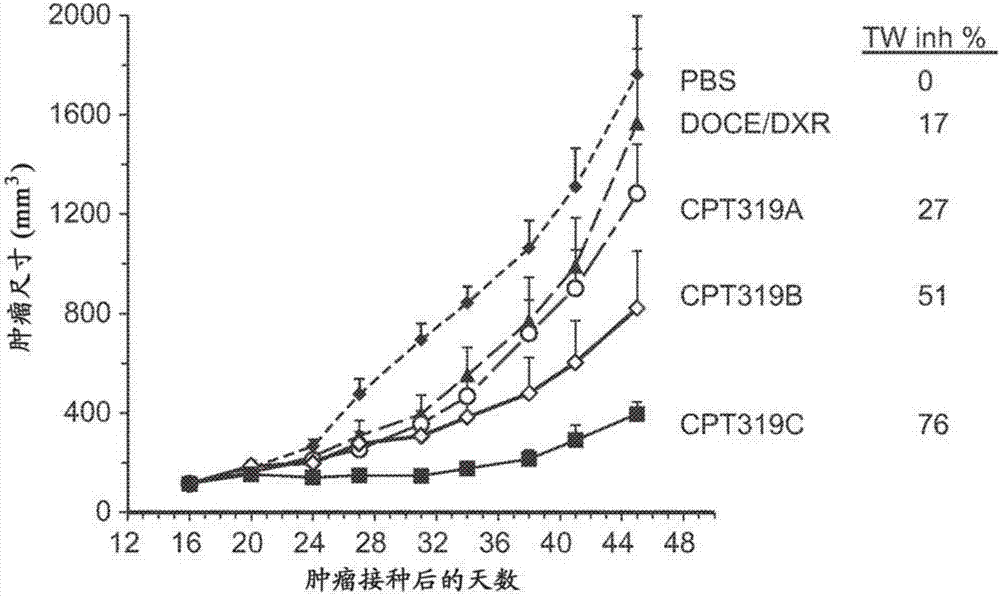
Examples
Embodiment 1
[0139] Embodiment 1: Preparation of liposome formulation CPT307C
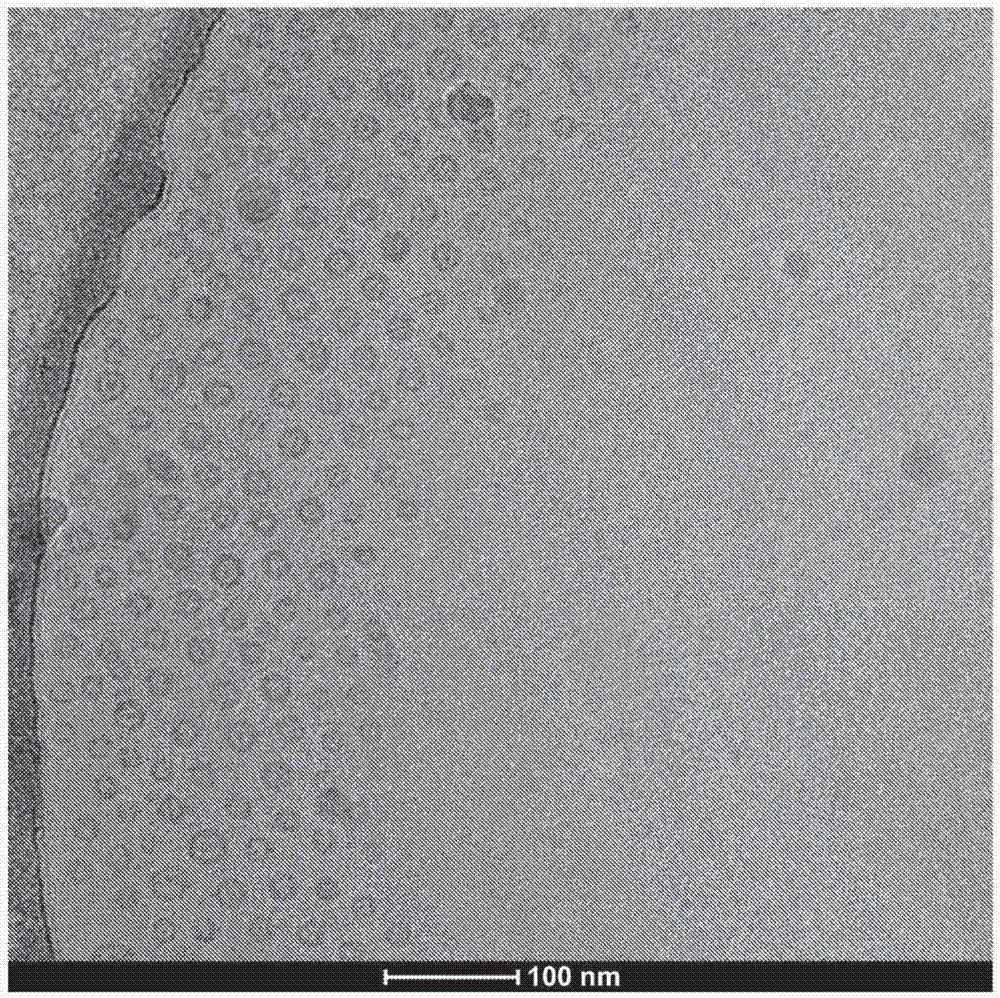
[0140] CPT307 contains the unsaturated lipids 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), cholesterol and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N -[methoxy9 polyethylene glycol)-2000] (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy9polyethyleneglycol)-2000]) (mPEG2000-DSPE). Unsaturated lipids were found to have a greater ability to encapsulate docetaxel compared to saturated lipids. The liposomal formulation CPT307B was prepared by dissolving 2100 mg DOPC, 280 mg cholesterol, 700 mg mPEG2000-DSPE and 175 mg docetaxel (DOCE) in 70 mL absolute ethanol. The composition (mol%) of the CPT307B lipid solution is shown in Table 1. In addition, three aqueous solutions of 250 mM ammonium sulfate (pH 6.5) were used. Twenty milliliters of each of the above four solutions were loaded into a 20 mL syringe. Each syringe is connected by tubing to the inlet of a five-port manifold. Thr...
Embodiment 2
[0145] Embodiment 2: Preparation of liposome formulation CPT308C
[0146] Different from CPT307C in Example 1, CPT308C contains polyunsaturated lipid L-α-phosphatidylcholine (soybean PC), which has a higher ability to encapsulate DOCE. Two milliliters of lipid / DOCE solution was prepared by dissolving 30 mg of soy PC, 10 mg of cholesterol, 10 mg of mPEG2000-DSPE and 6 mg of DOCE in absolute ethanol. The composition (mol %) of the lipid solution of the liposome formulation CPT308C is shown in Table 2. In addition, three 250 mM ammonium sulfate aqueous solutions (pH 6.5) were used. Two milliliters of each of the above four solutions were loaded into a 20 mL syringe. Each syringe is connected by tubing to the inlet of a five-port manifold. Through the tubing, the solution in the syringe is pumped by the syringe pump into the mixing chamber of the manifold. The liposome solution is expelled through the outlet and collected in a glass vial. The buffer was changed to histidine / s...
Embodiment 3
[0151] Embodiment 3: Preparation of liposome formulation CPT309C
[0152] Compared to CPT308C in Example 2, CPT309C contained a higher molar ratio of polyunsaturated lipid soybean PC, and thus showed a higher ability to encapsulate DEOCE. Two milliliters of lipid / DOCE solution was prepared by dissolving 30 mg of L-α-phosphatidylcholine (soybean PC), 4 mg of cholesterol, 10 mg of mPEG2000-DSPE and 6 mg of DOCE in absolute ethanol. The composition (mol %) of liposome preparation CPT309C lipid solution is shown in Table 3. In addition, three aqueous solutions of 250 mM ammonium sulfate (pH 6.5) were used. Two milliliters of each of the above four solutions were loaded into a 20 mL syringe. Each syringe is connected by tubing to the inlet of a five-port manifold. Through the tubing, the solution in the syringe is pumped by the syringe pump into the mixing chamber of the manifold. The liposome solution is expelled through the outlet and collected in a glass vial. The buffer wa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap