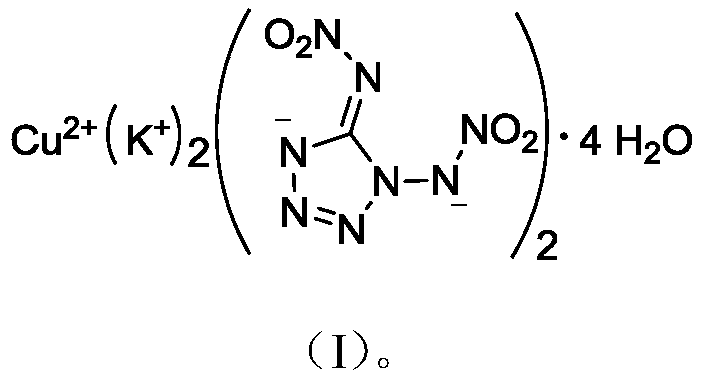
Bis(1,5-nitroaminotetrazole)-potassium cuprate tetrahydrate
A technology of nitroamine tetrazole and tetrahydrate, which is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., and can solve the problems of small detonation capacity and low detonation velocity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
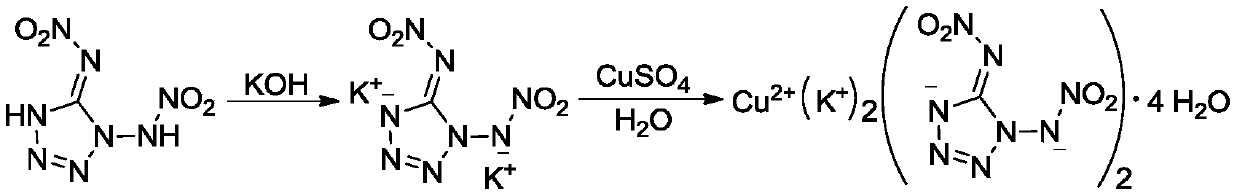
[0021] (1) Synthesis of 1,5-Dinitroaminotetrazole Potassium Salt
[0022] At room temperature, sequentially add 0.17g (0.89mmol) 1,5-dinitroaminotetrazole and 3.0mL methanol into the reaction flask, cool in an ice-water bath to 0-5°C, add dropwise 3.0mL containing 0.112g (2mmol) hydrogen The methanol solution of potassium oxide was continued to react at 0-5°C for 0.5 h, filtered, washed with methanol, and dried in vacuum to obtain 0.19 g of white solid with a yield of 79.8%.
[0023] Structure Identification:
[0024] Infrared Spectrum: IR(KBr,cm -1 ), υ: 1510, 1439, 1412, 1367, 1291, 1236, 1228, 1099, 1034, 912, 856
[0025] NMR spectrum: 13 C NMR (DMSO-d 6 ,125MHz), δ: 154.42
[0026] Elemental analysis: Molecular formula CK 2 N 8 o 4
[0027] Theoretical value: C 4.51, N 42.08
[0028] Found: C 4.62, N 42.03.
[0029] The above structural identification data confirmed that the obtained substance was indeed 1,5-dinitroaminotetrazole potassium salt.
[0030] (2) S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com