A kind of felodipine sustained-release tablet and preparation method thereof
A felodipine, gentle technology, applied in non-active ingredients medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of patient use risk, sudden release, uneven tablet coating, etc., achieve the effect of security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Preparation:
[0027] In step a, the raw material felodipine, the antioxidant propyl gallate, and the solubilizer polyoxyethylene hydrogenated castor oil are added to 95% ethanol solution to dissolve [felodipine: 95% ethanol=1:20 (g / ml)] to obtain Raw material solution; the binder hydroxypropyl cellulose was dissolved in 95% ethanol solution [binder: 95% ethanol = 1:15 (g / ml)] to obtain a binder solution;
[0028] In step b, the slow-release materials Hypromellose K15M and Hypromellose E50 are premixed uniformly by an equal amount addition method, and then mixed evenly with the excipient magnesium trisilicate by an equal amount addition method to obtain a mixture I; Lactose water and microcrystalline cellulose are premixed uniformly by equal addition method to obtain mixture II;
[0029] Step c, mix I and II in a three-dimensional mixer and mix evenly; transfer to a wet granulator after mixing, start mixing, add the raw material solution in step a, and shea...
Embodiment 2
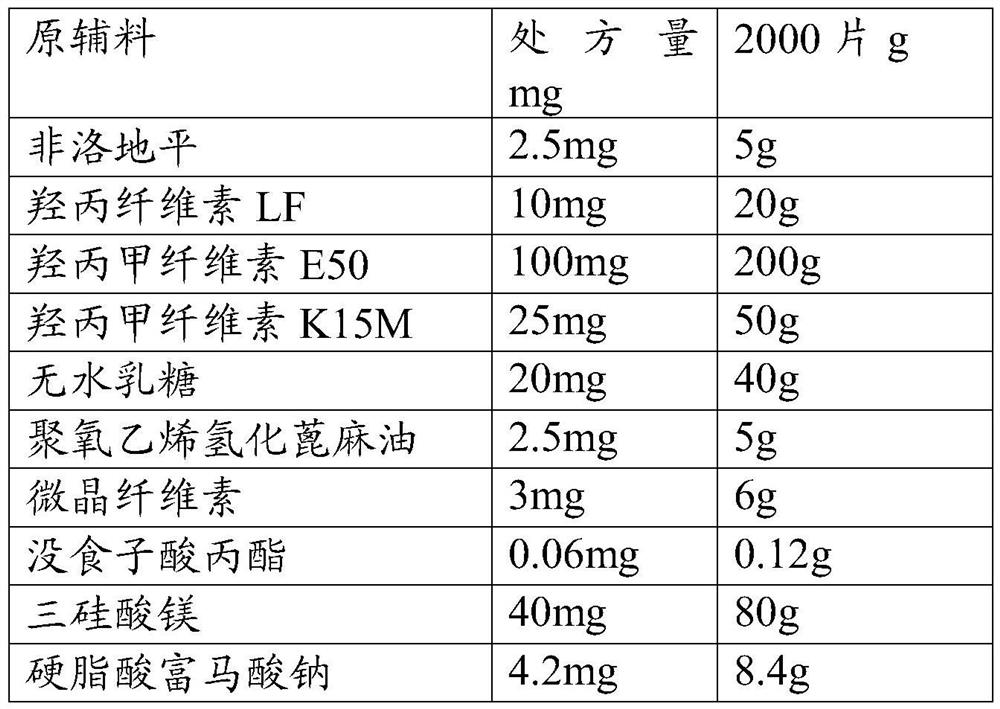
[0041] Raw materials Prescription amount mg 2000 tablets g Felodipine 5.0 mg 10g Hypromellose LF 10mg 20g Hypromellose E50 100mg 200g Hypromellose K15M 25mg 50g anhydrous lactose 20mg 40g Polyoxyethylene Hydrogenated Castor Oil 5.0 mg 10g microcrystalline cellulose 3mg 6g Propyl gallate 0.06mg 0.12g Magnesium trisilicate 40mg 80g Sodium stearate fumarate 3.9mg 8.4g
[0042] Preparation method: with embodiment 1
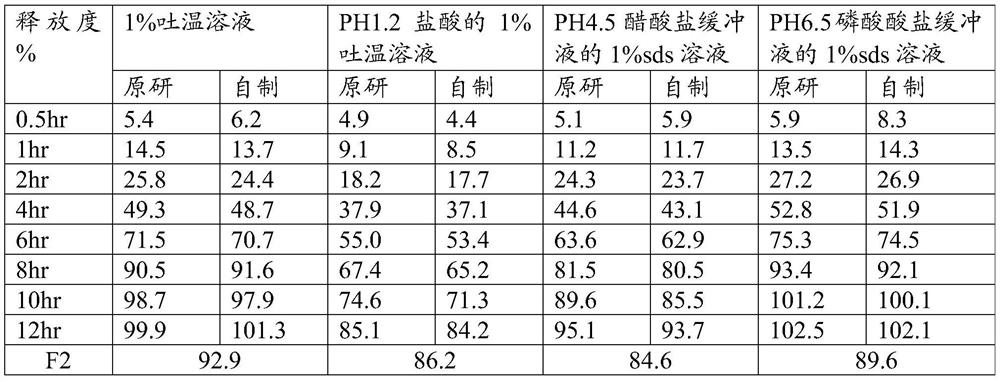
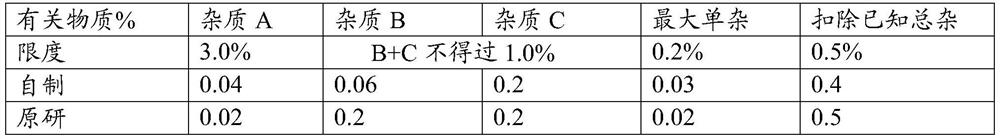
[0043] Taking the matching degree (F2) and related substances of the self-made sustained-release tablet in this embodiment and the release degree of the original preparation as the investigation index, the investigation results are shown in the following tables 3 and 4:
[0044] Table 3 Example 2 Sustained Release Tablets and the release of the original formulation compared to the results (specification 5.0mg)
[0045]
[0046] Table 4 Example 2 Sustained-release Tablet...
Embodiment 3
[0050] Raw materials Prescription amount mg 2000 tablets g Felodipine 10mg 20g Hypromellose LF 10mg 20g Hypromellose E50 100mg 200g Hypromellose K15M 25mg 50g anhydrous lactose 20mg 40g Polyoxyethylene Hydrogenated Castor Oil 10mg 20g microcrystalline cellulose 3mg 6g Propyl gallate 0.06mg 0.12g Magnesium trisilicate 40mg 80g Sodium stearate fumarate 4mg 8.4g
[0051] Preparation method: with embodiment 1
[0052] Taking the matching degree (F2) and related substances of the sustained-release tablets made in this embodiment and the release of the original preparation as the investigation indicators, the investigation results are shown in the following tables 5 and 6:
[0053] Table 5 Example 3 Sustained Release Tablets and the release result comparison of the original preparation (specification 10mg)
[0054]
[0055] Table 6 Example 3 Sustained Release Tablets and the comparison ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com