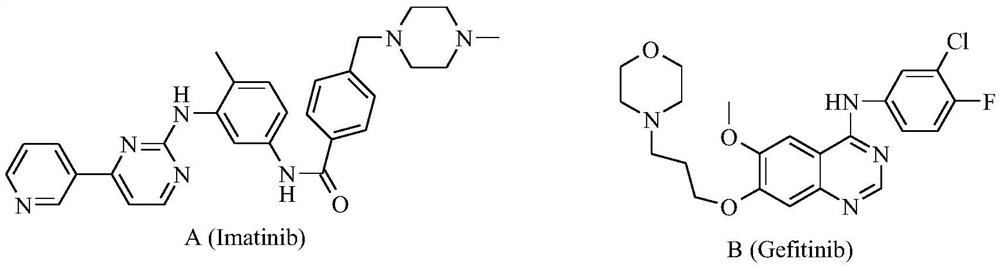
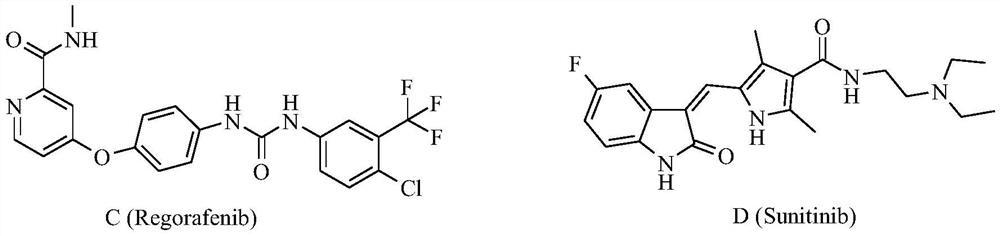
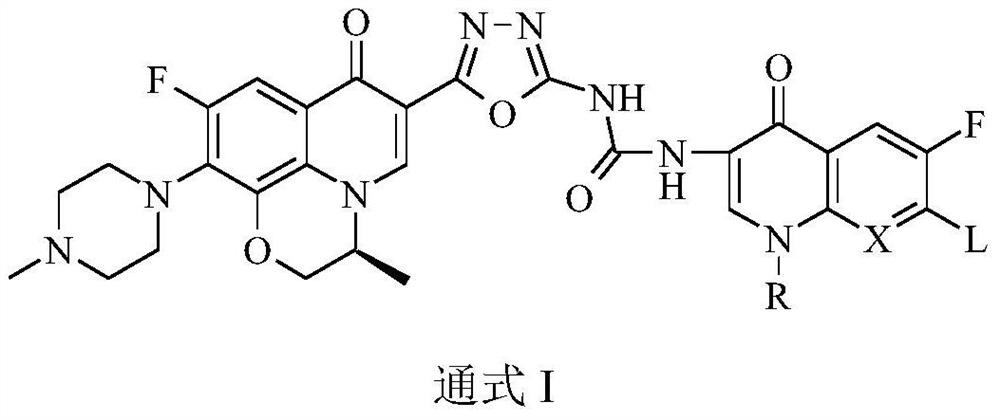
Bis-fluoroquinolone oxadiazole derivatives containing levofloxacin and its preparation method and application
A technology of fluoroquinolone-based oxadiazuron and levofloxacin, which is applied in the field of new drug discovery and innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The general method for the preparation of fluoroquinolone hydroxamic acid (1″-18″) is as follows: take the crude imidazolamide fluoroquinolone carboxylic acid (0.10mol) and suspend it in 500mL of pyridine, add 7.0-35.0g (0.1-0.50mol) of hydroxylamine hydrochloride , stirred in a water bath at 60-75°C for 8.0-24.0 hours, cooled to room temperature, collected the solid by filtration, washed the solid with pyridine, dried it in vacuum at 60-70°C, and dispersed it in saturated sodium bicarbonate solution (500mL) again. Stir in a water bath at 65°C for 3 to 5 hours, collect the solid by filtration, wash with deionized water until the pH is 7.0, and dry to obtain a crude product, which is washed with absolute ethanol (or anhydrous ethanol-DMF mixed solvent (V 乙醇 :V DMF =5:1) recrystallized to obtain analytically pure crystalline fluoroquinolone hydroxamic acid (1″~18″).
[0049] The preparation general method of two-fluoroquinolone base oxadiazole derivatives of target compo...
Embodiment 1
[0053] (S)-1-{2-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4(1H) -Keto-3-yl]-1,3,4-oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-( 1,3-oxypropyl)-quinoline-4(1H)-one-3-yl]-urea (I-1), its chemical structural formula is:
[0054]
[0055] The preparation method of the two-fluoroquinolone oxadiazuron of the present embodiment is: get ofloxacin hydroxamic acid (1 ") 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add CDI 0.50g (3.2mmol), Stir at room temperature until the material is dissolved. Then add 1.08g (2.7mmol) of levofloxacin C-3 oxadiazole amine II intermediate, and stir in a water bath at 55-60°C for 16 hours. Leave it overnight, collect the solid produced by filtration, and wash with acetonitrile. The crude product is washed with DMF -Recrystallization from a mixed solvent of ethanol to obtain a light yellow crystal (I-1), with a yield of 48%, m.p.216-218°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.57(brs,1H,NH),9.46(s,1H,NH),9.18,9....
Embodiment 2
[0057] (S, S)-1-{2-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4( 1H)-keto-3-yl]-1,3,4-oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1 -(1,3-oxopropyl)-quinoline-4(1H)-one-3-yl]-urea (I-2), its chemical structural formula is:
[0058]
[0059] The preparation method of the bis-fluoroquinolone oxadiazuron of the present embodiment is: take levofloxacin hydroxamic acid (2 ") 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add CDI 0.60g (3.7mmol), stir at room temperature until Material is dissolved. Then add levofloxacin C-3 oxadiazole amine II intermediate 1.08g (2.7mmol), water-bath 55~60 ℃ and stir 10 hours.Stir overnight, filter the solid that produces, acetonitrile washing.Crude ethanol recrystallization, A light yellow crystal (I-2) was obtained with a yield of 42%, m.p.207-209°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.62(brs,1H,NH),9.50(s,1H,NH),9.23,9.16(2s,2H,2×2′-H),8.26~7.83(m,2H,2×5′- H),4.97~4.83(m,6H,2×OCH 2 CHN),3.56~3.45(m,8H,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com