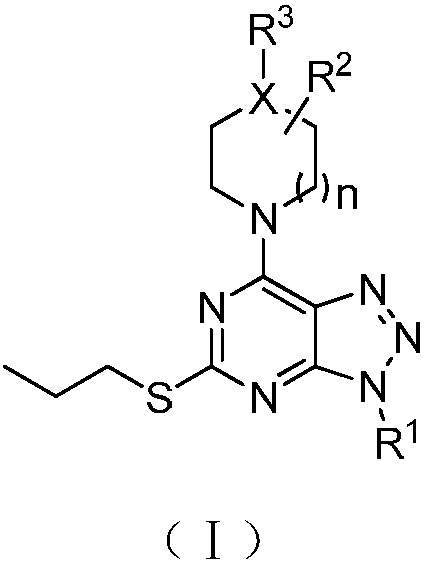
Pyrimidotriazole compounds, preparation method and application thereof
A compound, triazole technology, applied in the field of medicine, can solve problems such as low selectivity and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0073]
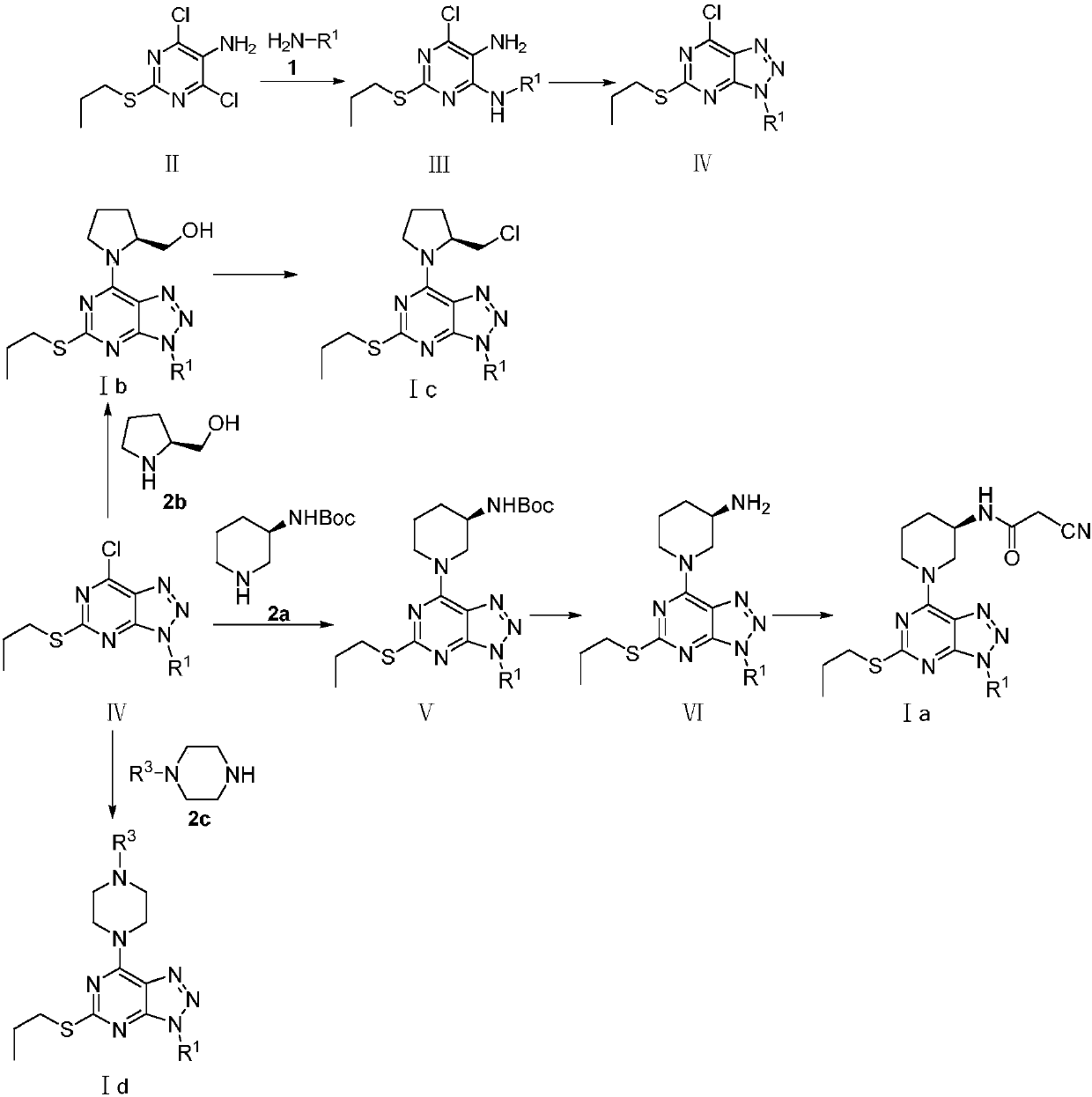
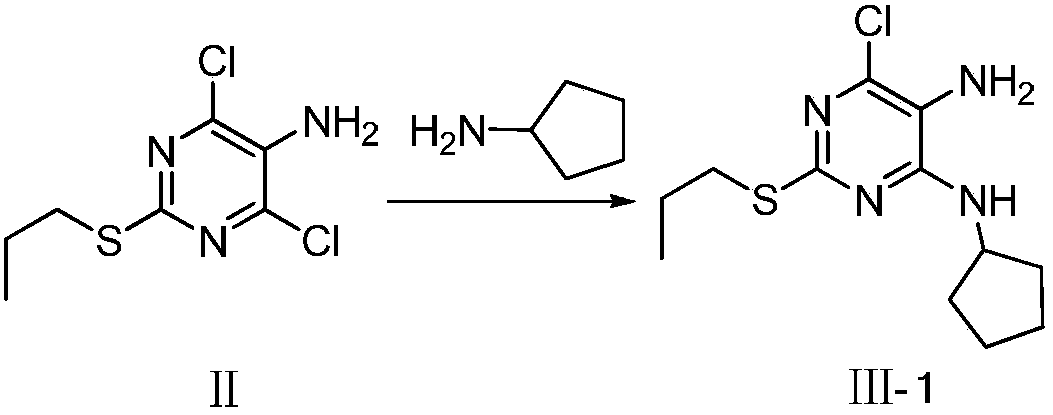
[0074] Referring to the synthesis method of Reference Example 1, the key intermediate III-2 was synthesized by replacing cyclopentylamine with benzylamine.
[0075] 1 H NMR (400MHz, CDCl 3 )δ: 0.97(t, J=7.6Hz, 3H), 1.64-1.73(m, 2H), 3.01(t, J=7.2Hz, 2H), 3.14-3.19(m, 3H), 4.67(d, J =4.0Hz, 2H), 5.92(s, 1H), 7.27-7.33(m, 5H).
Embodiment 3
[0077]
[0078] Referring to the synthesis method of Reference Example 1, the key intermediate III-3 was synthesized by replacing cyclopentylamine with methylamine hydrochloride.
[0079] 1 H NMR (400MHz, CDCl 3 )δ: 1.02(t, J=7.2Hz, 3H), 1.69-1.79(m, 2H), 3.02-3.08(m, 5H), 3.21(s, 2H), 5.38(s, 1H).
[0080] Reference Example 4:
[0081]
[0082] Add 28.6g of compound III-1 and 200ml of acetonitrile into the reaction flask, add 24.02g of acetic acid at 5-10°C and stir, dropwise add 8.30g of NaNO 2 80ml of the aqueous solution, dropwise, add 20ml of ethyl acetate, continue to stir for 1h, then pour the reaction solution into 300ml of distilled water, extract with ethyl acetate, dry the organic phase with anhydrous magnesium sulfate, concentrate under reduced pressure, and the residue with n-hexane - Refined with ethyl acetate to obtain 26.15 g of brown needle-like solid IV-1. 1 H NMR (400MHz, CDCl 3)δ: 1.08(t, J=7.2Hz, 3H), 1.77-1.86(m, 4H), 2.00-2.09(m, 2H), 2.27-2...
Embodiment 1
[0090] (R)-N-(1-(3-cyclopentyl-5-(propylthio)-3H-[1,2,3]triazol[4,5-d]pyrimidin-7-yl) Preparation of piperidinyl-3-yl)-3-cyanoacetamide (compound Ⅰa-1)
[0091]
[0092] Add 1g (R)-3-tert-butoxycarbonylaminopiperidine, 1.5g compound IV-1, 0.8K 2 CO 3 React with 50ml of DMF at 60°C for 8h, TLC controls the completion of the reaction, pour the reaction solution into 150ml of cold water, extract with ethyl acetate (100ml×3), dry the organic phase with anhydrous magnesium sulfate, filter, evaporate the organic solvent under reduced pressure, Compound V-1 was obtained by column chromatography.
[0093] Add 50mLCH to a reaction flask equipped with a thermometer and stirring device 2 Cl 2 and 20ml of trifluoroacetic acid, stir evenly, add 2.3g of intermediate Ⅴ-1 to the mixture, react at 30°C for 3h, TLC controls the reaction to be complete, concentrate under reduced pressure, add 1.5g of DBU, 1.15g of ethyl cyanoacetate to the residue Ester and 50ml of n-butanol, stirred, re...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap