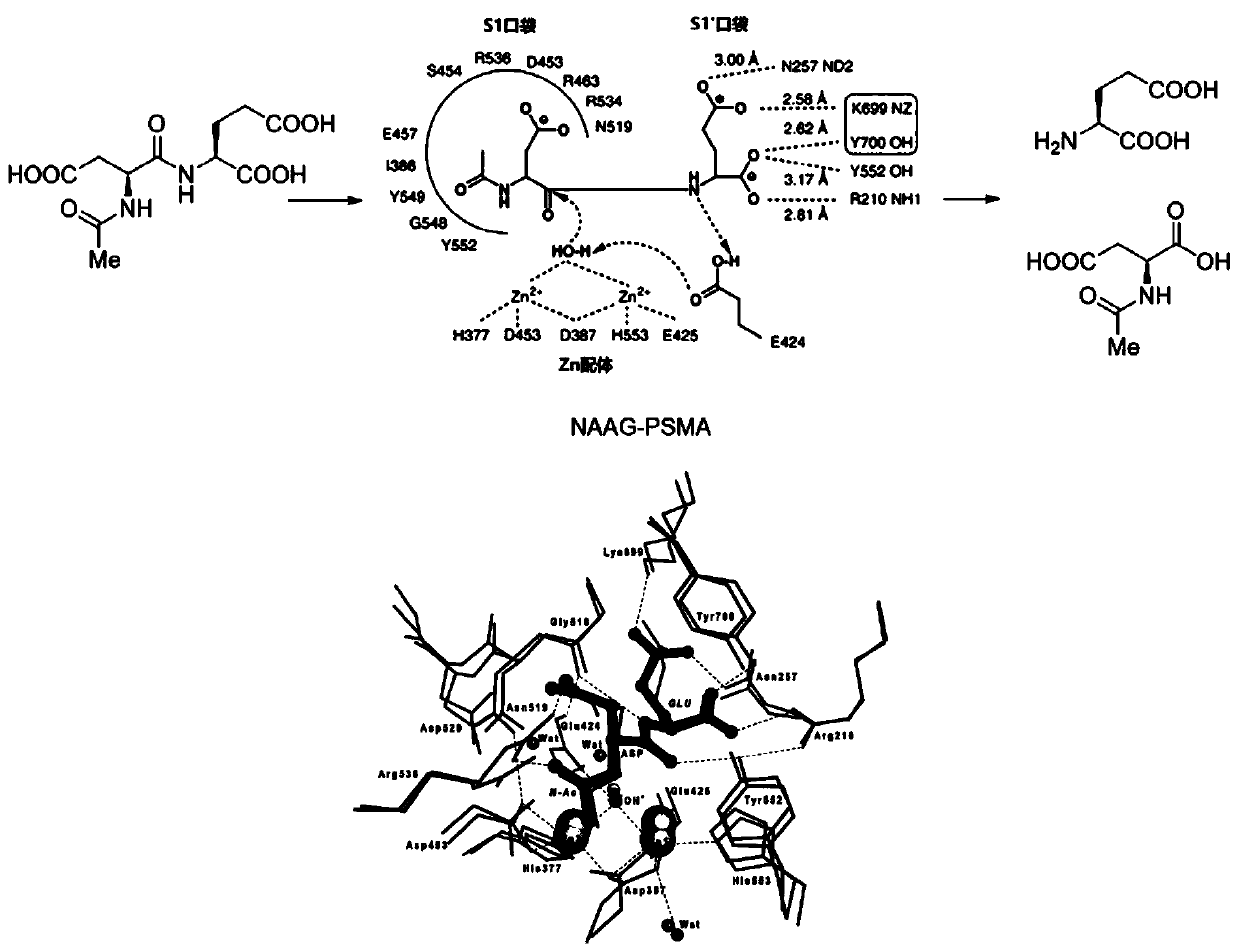
A kind of psma inhibitor, compound and application
A compound and drug technology, applied in the field of PSMA inhibitors and PSMA inhibitor compounds with functional groups, can solve the problems of decreased binding constant, few application prospects, unstable compound structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
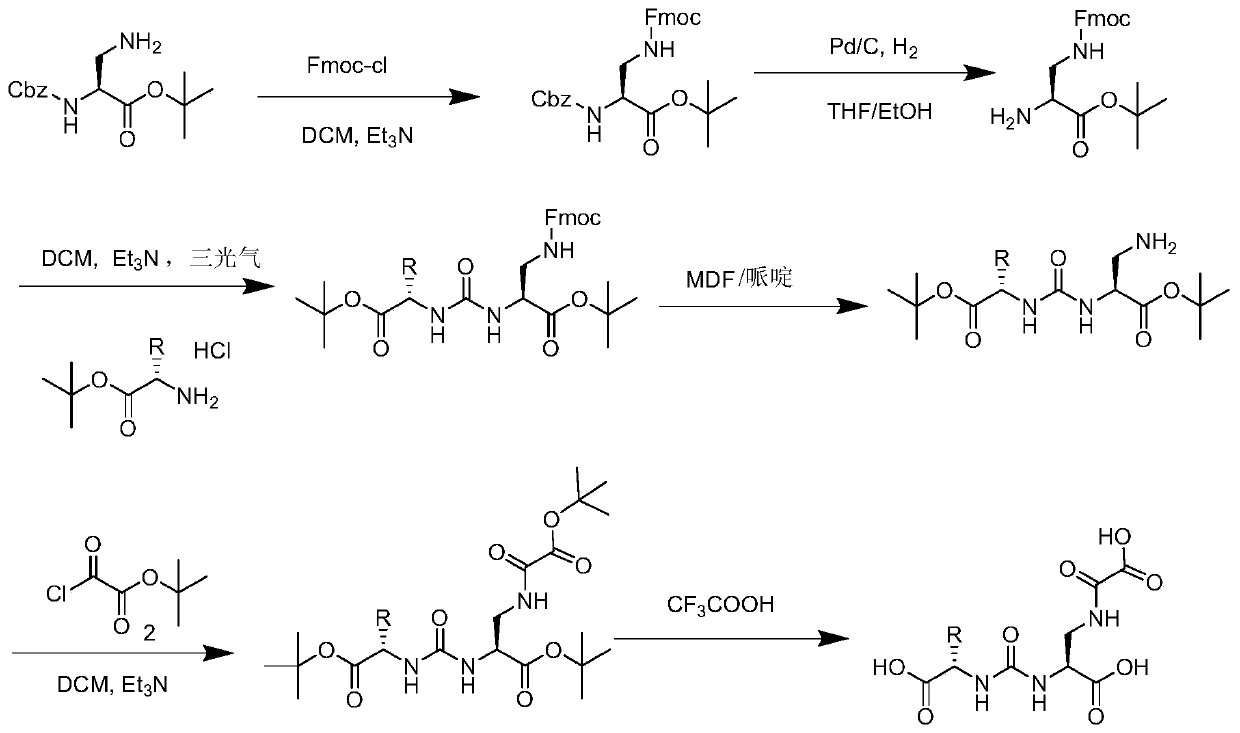
Image
Examples
Embodiment 1-2
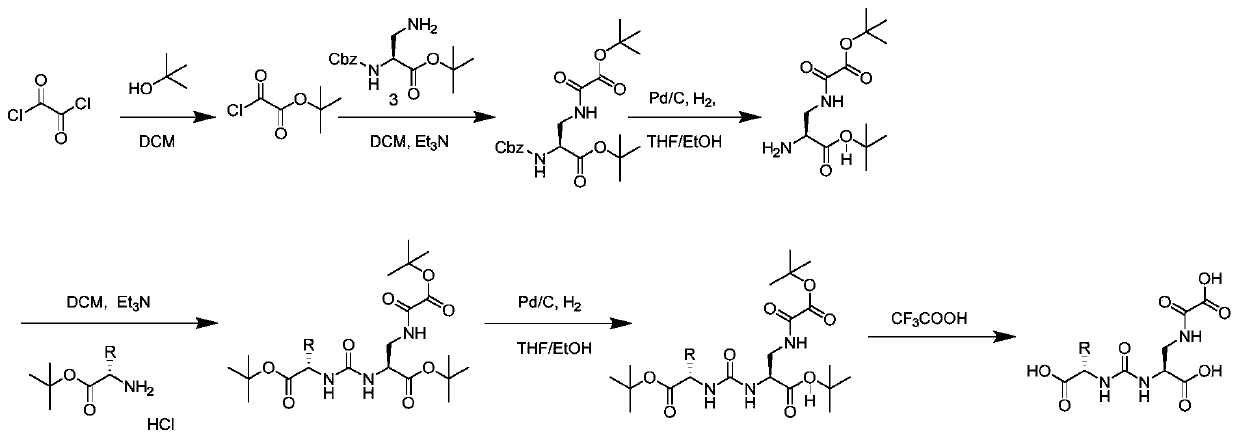
[0083] This example is used to illustrate the synthesis and characterization of compound S1 and compound S2. Synthetic route such as Figure 4 shown.
[0084] (1) Synthesis of compound 2 tert-butyl 2-chloro-2-oxoacetate:
[0085] In a 100mL round-bottomed flask, oxalyl chloride (1g, 7.88mmol) was dissolved in anhydrous dichloromethane (15mL), and tert-butanol (584mg, 7.88mmol) was dissolved in anhydrous dichloromethane (15mL) under ice-cooling stirring. The solution was slowly added dropwise into the reaction liquid, and after the dropwise addition was completed, the reaction was carried out at room temperature under nitrogen protection for 24 hours, and the solvent was removed under reduced pressure to obtain a colorless liquid product 2, which was directly used in the next reaction.
[0086] (2) Synthesis of compound 4 (S)-tert-butyl 2-(((benzyloxy)carbonyl)amino)-3-(2-(tert-butoxy)-2-oxoacetamido)propanoate:
[0087] In a 100mL round bottom flask, compound 3 (1g, 3.40mmo...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap