Pharmaceutical combinations of histone deacetylase inhibitor and proteasome inhibitor or immunomodulatory drug for the treatment of hematological cancer
A technology for proteasome inhibitors and immunomodulatory drugs, which are used in drug combinations, enzyme inhibitor components, active ingredients of boron compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
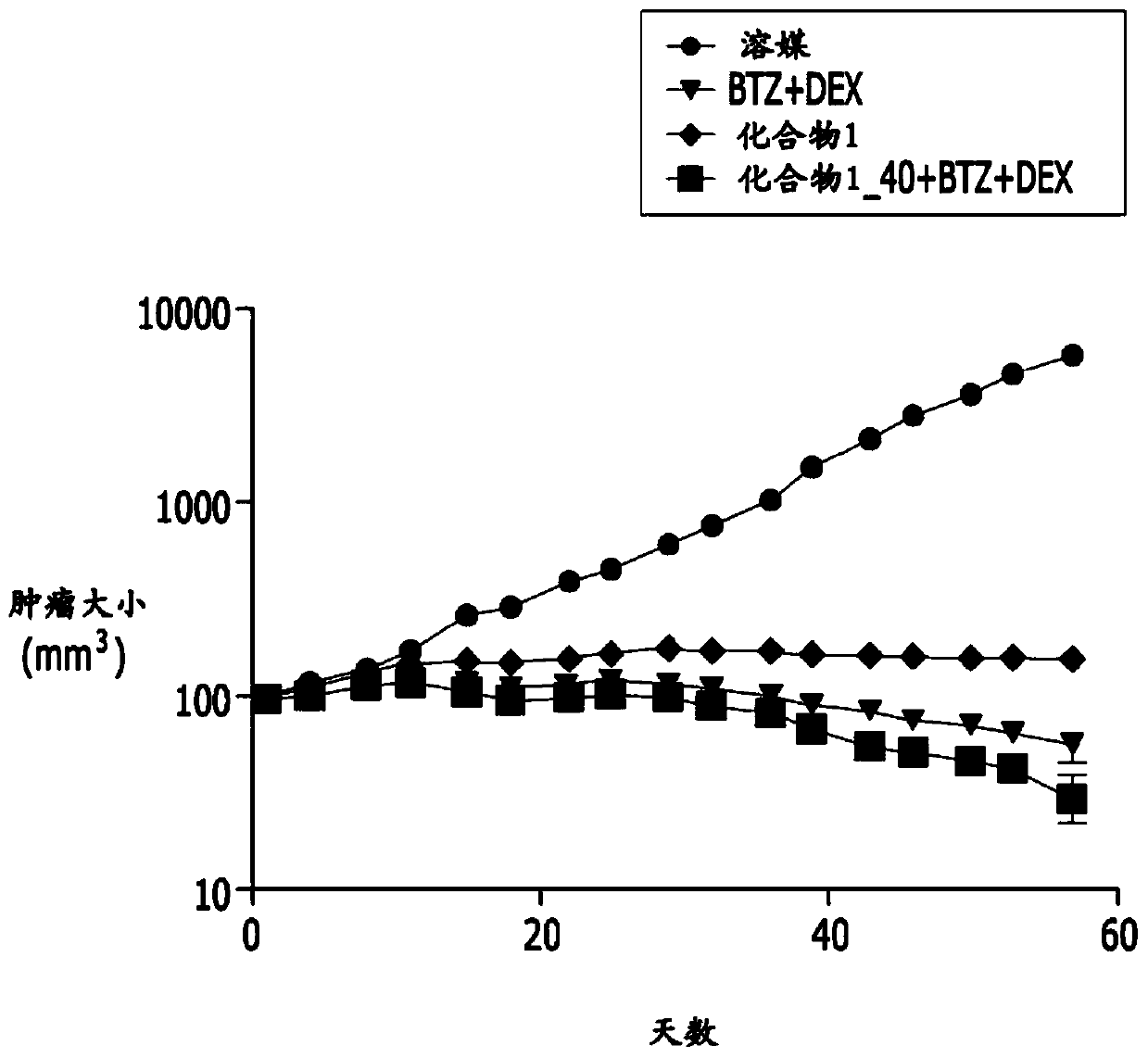
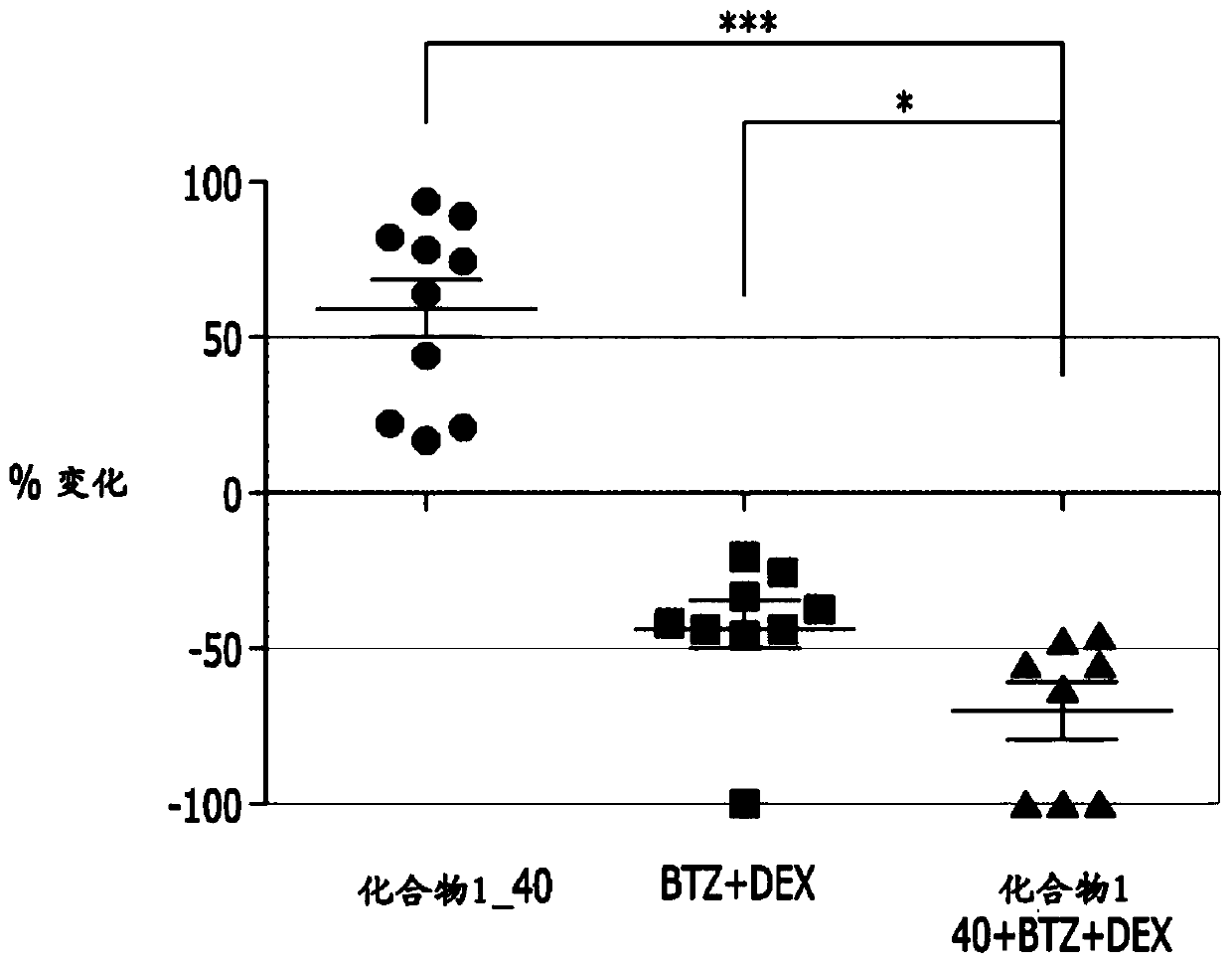
Embodiment 1
[0046]
[0047] 1-1) Experimental method
[0048] a. Preparation of animal tumor models
[0049] The human multiple myeloma cell line MM.1s was purchased from ATCC (USA). The MM.1s cell line was maintained with RPMI1640 (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA).
[0050] MM.1s cells mixed with Matrigel were subcutaneously administered to NOD.CB17-Prkdcscid / NCrHsd male mice (2×10 7 cells / head), grouped to make the tumor size evenly distributed, and then used in the experiment.
[0051] b. Preparation of active ingredients
[0052] The compound of Chemical Formula 1 was prepared at a concentration of 4 mg / ml by dissolving with a physiological saline solution.
[0053] Bortezomib was prepared at a concentration of 0.005 mg / ml by dissolving with physiological saline solution.
[0054] Dexamethasone was prepared at a concentration of 0.05 mg / ml by diluting the 5 mg / mL stock solution with physiological saline solution.
[...
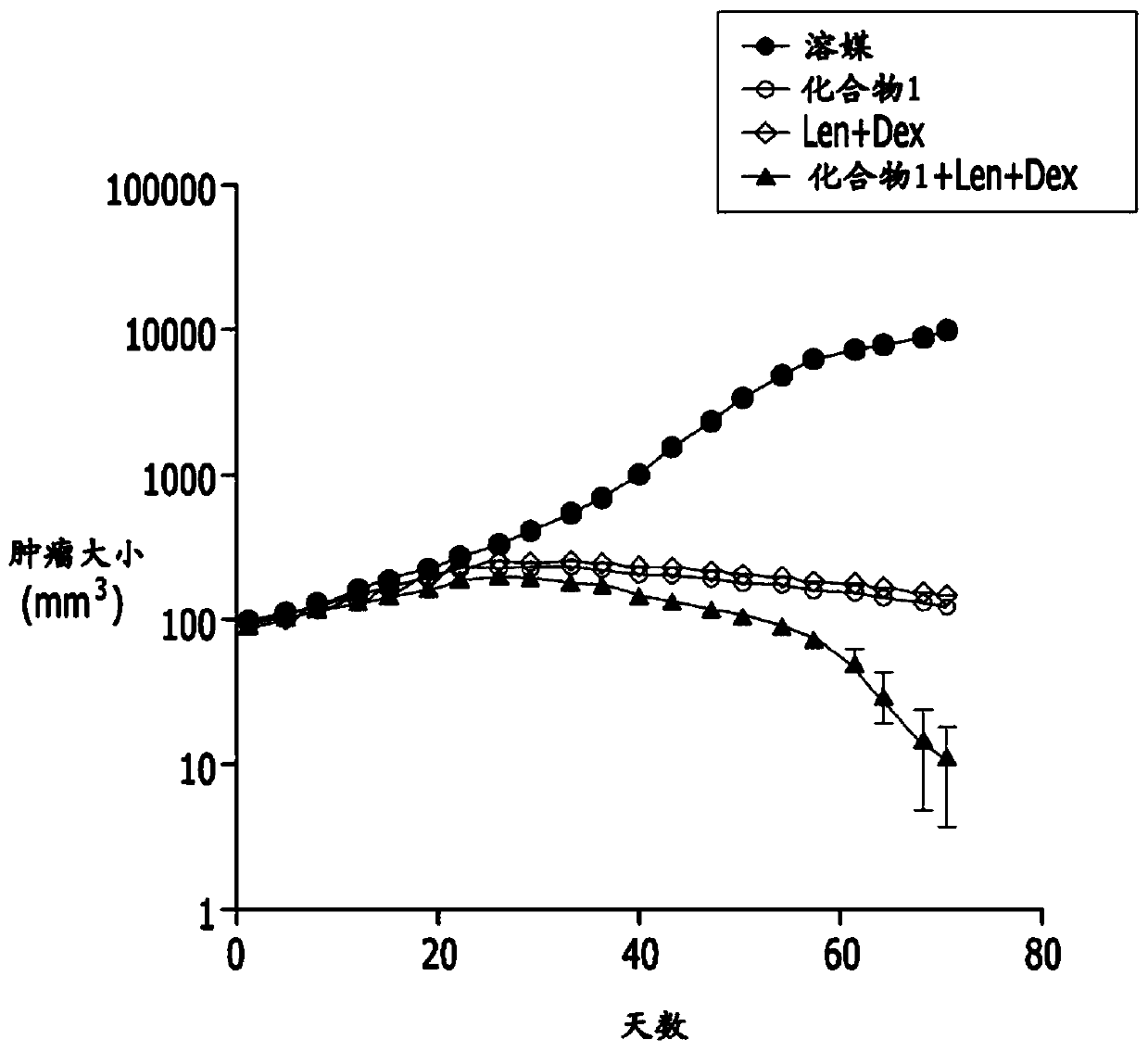
Embodiment 2
[0072]
[0073] 2-1) Experimental method
[0074] a. Preparation of animal tumor models
[0075] The human multiple myeloma cell line MM.1s was purchased from ATCC (USA). The MM.1s cell line was maintained with RPMI1640 (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA).
[0076] MM.1s cells mixed with Matrigel were subcutaneously administered to NOD.CB17-Prkdcscid / NCrHsd male mice (2×10 7 cells / head), grouped to make the tumor size evenly distributed, and then used in the experiment.
[0077] b. Preparation of active ingredients
[0078] The compound of Chemical Formula 1 was prepared at a concentration of 6 mg / ml by dissolving with a physiological saline solution.
[0079] Lenalidomide was prepared at a concentration of 1 mg / ml by dissolving 1% HCl in PBS and then titrating to neutral pH.
[0080] Dexamethasone at a concentration of 0.2 mg / ml was prepared by diluting 5 mg / ml dexamethasone with physiological saline.
[0081] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com