A kind of stable ivabradine hydrochloride tablet and preparation method thereof
A technology of ivabradine hydrochloride and ivabradine hydrochloride, which is applied in the field of ivabradine hydrochloride tablets and its preparation, can solve the problems of related substances exceeding the standard, and achieve the problems of improving stability, ensuring quality, and overcoming crystal transformation of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
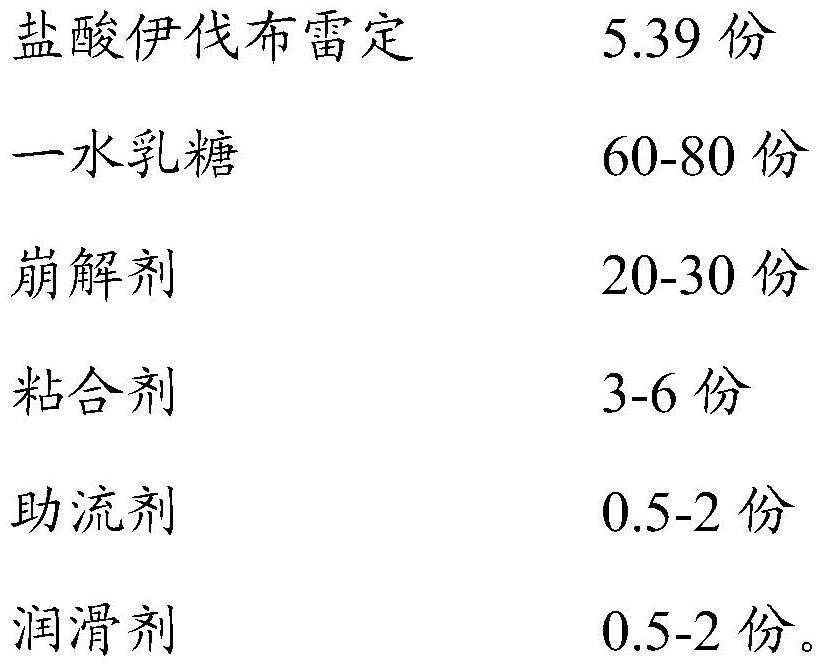
[0035] Prescription for ivabradine hydrochloride tablets
[0036]
[0037] The preparation method is as follows:
[0038] Raw material drug pretreatment: the raw material drug is crushed and passed through a 100-mesh sieve;
[0039] Weighing: Weigh each raw material and auxiliary material according to the weight ratio;
[0040] Mixing and granulation: add lactose monohydrate and corn starch into the wet granulator, pre-mix for 7 minutes, add an appropriate amount of purified water, mix and granulate for 3-5 minutes, make soft materials and sieve;
[0041] Drying and granulation: Dry the soft material at 60-65°C until the moisture content is below 3%.
[0042] Blending and tableting: Add dry granules, ivabradine hydrochloride, maltodextrin, colloidal silicon dioxide and magnesium stearate into a three-dimensional blender and blend for 10 minutes. After passing the test, select a φ8.5*4.6 special-shaped punch, and press the tablet according to the prescribed tablet weight....
Embodiment 2
[0045] Prescription for ivabradine hydrochloride tablets
[0046]
[0047] The preparation method is as follows:
[0048] Raw material drug pretreatment: the raw material drug is crushed and passed through a 100-mesh sieve;
[0049] Weighing: Weigh each raw material and auxiliary material according to the weight ratio;
[0050] Mixing and granulation: add lactose monohydrate and microcrystalline cellulose into the wet granulator, pre-mix for 7 minutes, add a certain amount of purified water, mix and granulate for 3-5 minutes, make soft materials and sieve;
[0051] Drying and granulation: Dry the soft material at 60-65°C until the moisture content is below 3%.
[0052] Blending and tableting: Add dry granules, ivabradine hydrochloride, sodium carboxymethyl starch, colloidal silicon dioxide and magnesium stearate into a three-dimensional blender and blend for 10 minutes. After passing the test, select a φ8.5*4.6 special-shaped punch, and press the tablet according to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com