Method for preparing lyral for cosmetic testing
A technology of lyral and crude products, which is applied in the detection of cosmetics, especially the detection of allergenic fragrances in cosmetics, can solve the problems of inconvenient use, expensive reference substances, and difficulty in obtaining them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Add 539g (3.5mol) myrcenol and 0.539g hydroquinone in the 1000mL four-necked flask that thermometer, mechanical stirring device and reflux condenser are equipped with, be heated to 110 ℃; In described myrcenol, dropwise add 235g ( 4.2 mol) of acrolein was added dropwise for 6 hours. During the dropwise addition, the reaction bottle was placed in a freezing tank, and the temperature of the reaction bottle was kept at 110°C to 130°C; after the dropwise addition of acrolein was completed, the reaction was continued for 1 hour to obtain mixture;
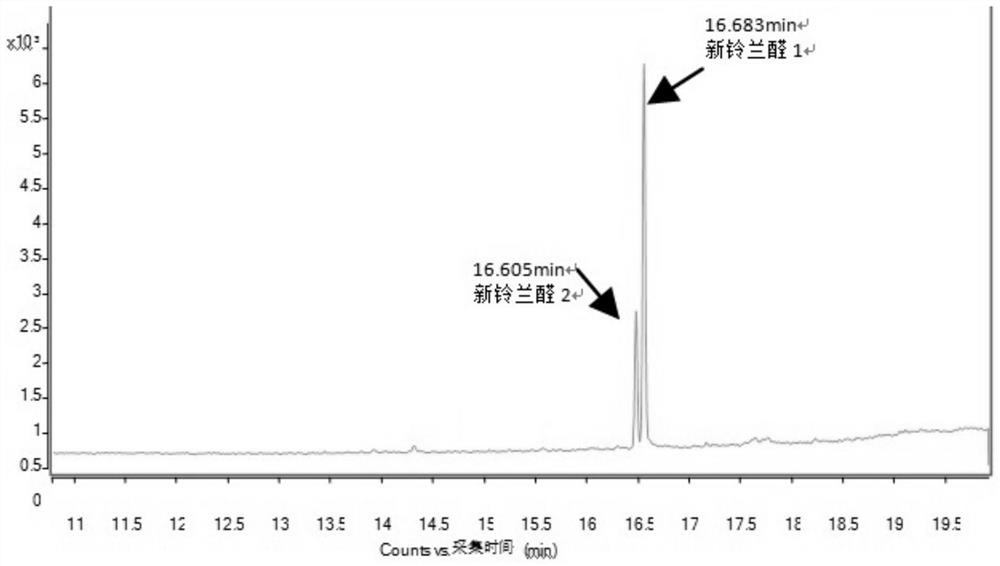
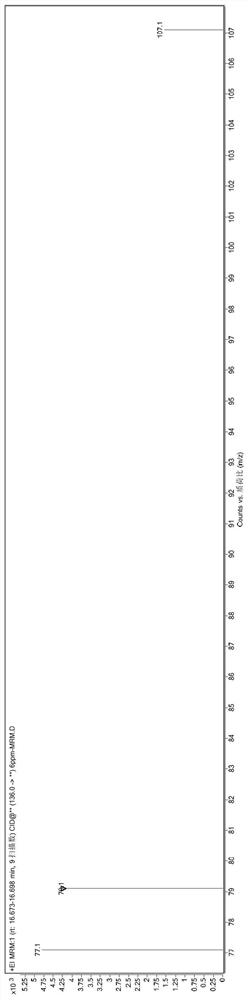
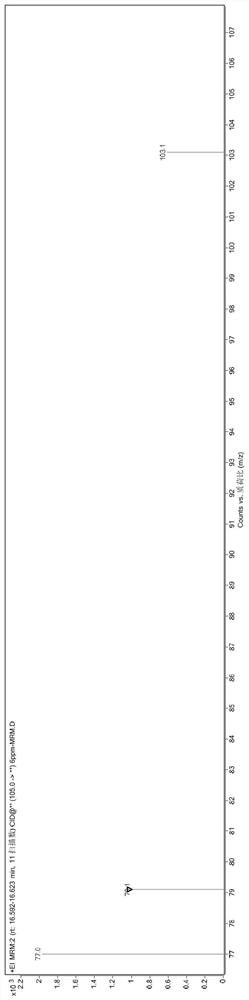
[0096] The reaction mixture was washed three times with 200g deionized water to obtain 766g lyral crude product (GC recorded lyral content 94.32%); the crude product was rectified below vacuum tightness 2mmHg, and collected at 120° C. The fraction between 125° C. obtained 611 g of lyral (the content of lyral was 99.64% as measured by GC).
[0097] The obtained lyral fraction (about 1 / 4 amount, multiplied by the remainder for late...
Embodiment 2
[0099]Add 539g (3.5mol) myrcenyl alcohol and 0.539g hydroquinone in the 1000mL four-necked flask that thermometer, mechanical stirring device and reflux condenser are equipped with, be heated to 110 ℃; In described myrcenyl alcohol, dropwise add 216g ( 3.86 mol) of acrolein, added dropwise for 6 hours. During the dropwise addition, the reaction bottle was placed in a freezing tank, and the temperature of the reaction bottle was kept at 110°C to 130°C; after the dropwise addition of acrolein was completed, the reaction was continued for 1 hour to obtain mixture;
[0100] The reaction mixture was washed three times with 180g deionized water to obtain 757g lyral crude product (94.73% lyral content was recorded by GC); The fraction between 125° C. obtained 607 g of lyral (the content of lyral was 99.73% as measured by GC).
[0101] The obtained lyral fraction (about 1 / 4 amount, multiplied by the remainder for later use) is introduced into the sodium chloride powder that can pass ...
Embodiment 3
[0103] Add 539g (3.5mol) myrcenol and 0.539g hydroquinone in the 1000mL four-necked flask that thermometer, mechanical stirring device and reflux condenser are equipped with, be heated to 110 ℃; In described myrcenol, dropwise add 196g ( 3.5 mol) of acrolein was added dropwise for 6 hours. During the dropwise addition, the reaction bottle was placed in a freezing tank, and the temperature of the reaction bottle was kept at 110°C to 130°C; after the addition of acrolein was completed, the reaction was continued for 1 hour to obtain the reaction mixture;
[0104] The reaction mixture is washed three times with 180g deionized water to obtain 748g lyral crude product (GC records 88.76% lyral content); The fraction between 125° C. obtained 503 g of lyral (the content of lyral was 99.46% as measured by GC).
[0105] The obtained lyral fraction (about 1 / 4 amount, multiplied by the remainder for later use) is imported into the sodium chloride powder that can pass through a 200 mesh s...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap