A kind of anthracene derivative and its preparation method and application
An anthracene derivative and reaction technology, applied in the field of anthracene derivatives and their preparation, can solve the problems of insufficient carrier injection and transport, and the need to improve the fluorescence quantum efficiency of solid-state thin film aggregated state, so as to be beneficial to factory production. preparation, beneficial to popularization and application, and the effect of improving fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
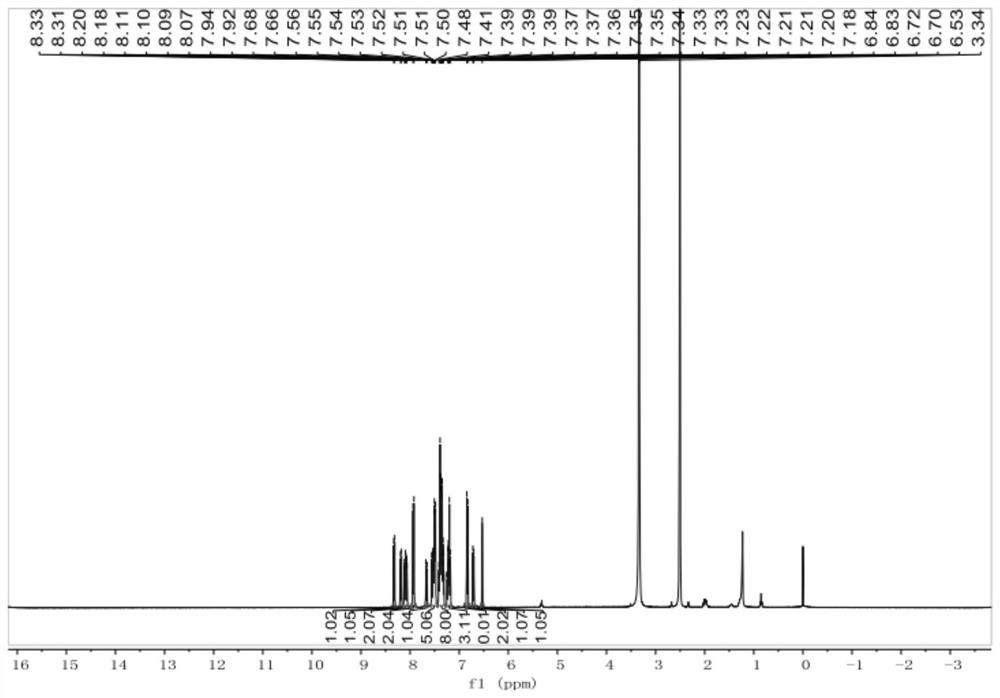
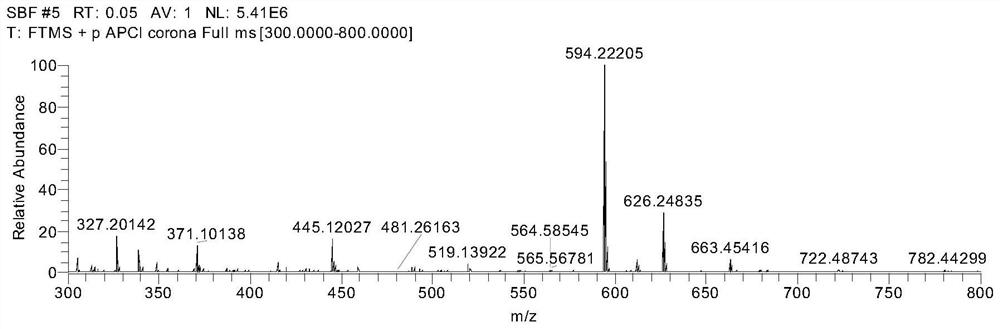
[0066] Example 1 Preparation of 4-(10-(9,9'-spirobis[fluorene]-2-yl)anthracen-9-yl)benzonitrile
[0067] Step 1: Preparation of 4-(10-bromoanthracen-9-yl)benzonitrile:
[0068] Add 9,10-dibromoanthracene (1.67g, 5mmol), 4-cyanophenylboronic acid (0.74g, 5mmol), and tetrakistriphenylphosphine palladium (0.115g, 0.1mmol) into a 250mL two-necked flask in sequence, and The flask was evacuated under vacuum and replaced three times under dry nitrogen, then 40 mL of toluene was added and 10 mL of saturated K 2 CO 3 aqueous solution. The reaction was heated to reflux at 90° C. and stirred for 12 hours. Extract with saturated brine and dichloromethane. Distilled under reduced pressure to obtain a yellow solid, using silica gel powder as a stationary phase and petroleum ether / dichloromethane as an eluent, 0.89 g of a white powder was obtained by column chromatography (yield 50%).
[0069] The reaction equation is as follows:
[0070]
[0071] Step 2: Preparation of 2-boryl-9,9'...
Embodiment 2
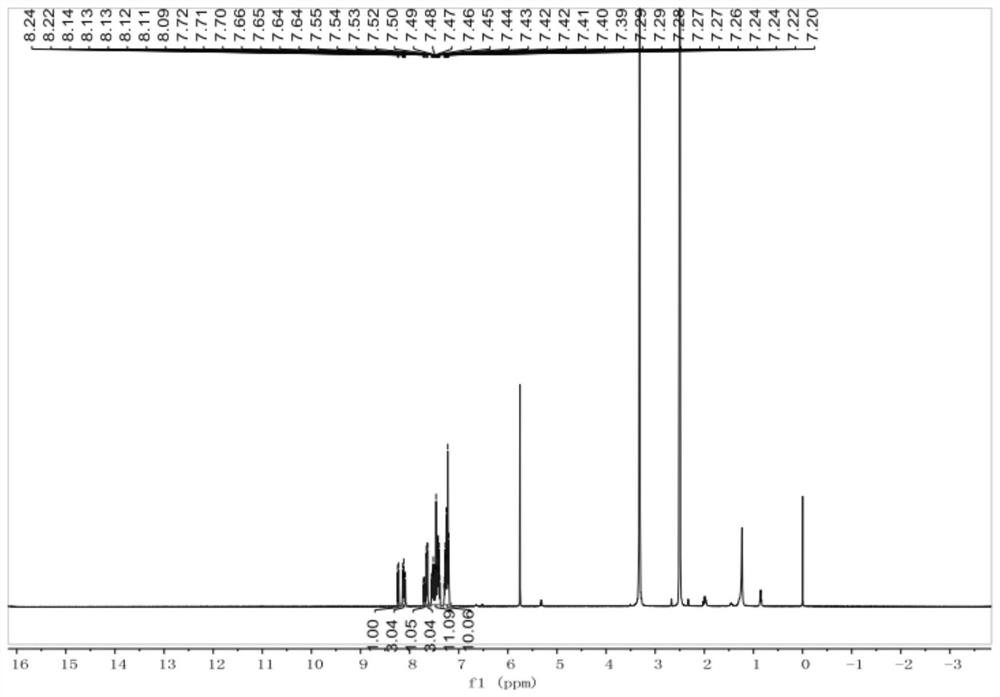
[0082] Example 2 Preparation of 4-(10-(9,9-diphenyl-9H-fluoren-2-yl)anthracen-9-yl)benzonitrile
[0083] Step 1 of the present embodiment is the same as embodiment 1, and step 2 and step 3 are as follows:
[0084] Step 2: Preparation of 2-boryl-9,9-diphenyl-9H-fluorene
[0085] 2-Bromo-9,9-diphenyl-9H-fluorene (1.18g, 3mmol), biboronic acid pinacol ester (1.02g, 4mmol), catalyst [1,1'-bis(diphenylphosphine) Ferrocene] palladium dichloride dichloromethane complex (0.073, 0.09mmol), potassium acetate (0.882g, 9mmol) were successively added to a 100mL two-necked flask, and the flask was evacuated under vacuum and replaced three times in dry nitrogen , then 1,4-dioxane (20 mL) was added. After stirring the reaction at 85°C for 8 hours, it was extracted with saturated brine and dichloromethane. Distilled under reduced pressure to obtain a black solid, using silica gel powder as a stationary phase and petroleum ether / dichloromethane as an eluent, 1.02 g of a white oil was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com