Anti-pd-1 antibodies and methods of making and using thereof
A PD-1 and monoclonal antibody technology, applied in the fields of botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Production of anti-PD-1 antibodies
[0092] Development of monoclonal antibodies against human PD-1 by immunizing New Zealand white rabbits. Initial immunizations were performed subcutaneously with 100 μg recombinant human PD-1 ectodomain mixed 1:1 v / v in complete Freund's adjuvant. This was followed by booster immunizations at 3, 6, 9 and 8 weeks with 50 μg of antigen in incomplete Freund's adjuvant. In addition to antigen, at weeks 3, 6 and 8, 1X10 6 Animals were boosted with HEK-293 cells that were transiently transfected to express full-length human PD-1.
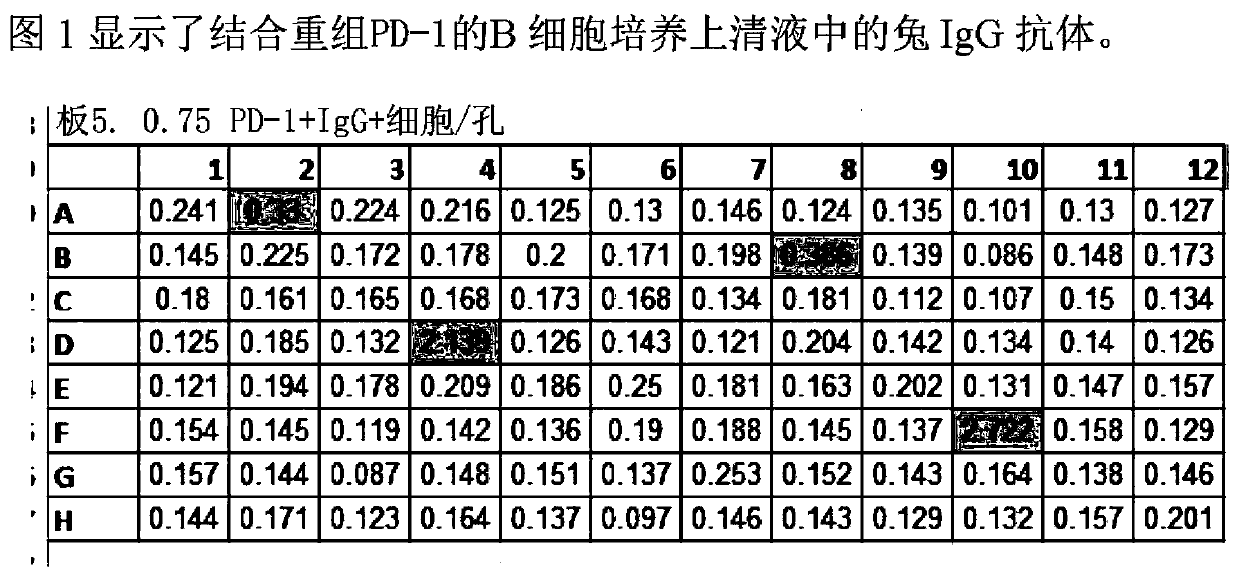
[0093] At week 8, sera from animals were tested for anti-PD-1 titers by ELISA. 96-well plates were passively adsorbed overnight at 4°C with goat anti-rabbit IgG antibody (Jackson ImmunoResearch). The coated wells were washed and blocked with 1% milk for 1 hour at room temperature, and then incubated with human PD-1 extracellular domain-human Fc domain fusion protein for 1 hour at room temperature...
Embodiment 2
[0101] Example 2): Binding affinity of anti-PD-1 antibodies
[0102] Binding kinetics of selected anti-PD-1 antibodies AB1-AB5 and AB1HU-AB5HU were determined by biolayer interferometry on a Fortebio Octbe Red 96 instrument. First, purified antibodies were produced by protein A chromatography using antibody supernatants transiently transfected with HEK-293.
[0103] The assay was performed by immobilizing purified antibodies onto anti-human Fc biosensors. The association and dissociation of PD-1 to the biosensor was then observed at various concentrations of PD-1. Specifically, eight anti-human Fc biosensors were placed in wells containing the same purified antibody for 5 minutes. The biosensor was equilibrated for 1 min in kinetic buffer (Pall Fortebio) to establish a baseline. PD-1 association was observed by placing the biosensor in wells containing various concentrations of human PD-1 ectodomain for 5 min. Dissociation was measured after transferring the biosensor to...
Embodiment 3
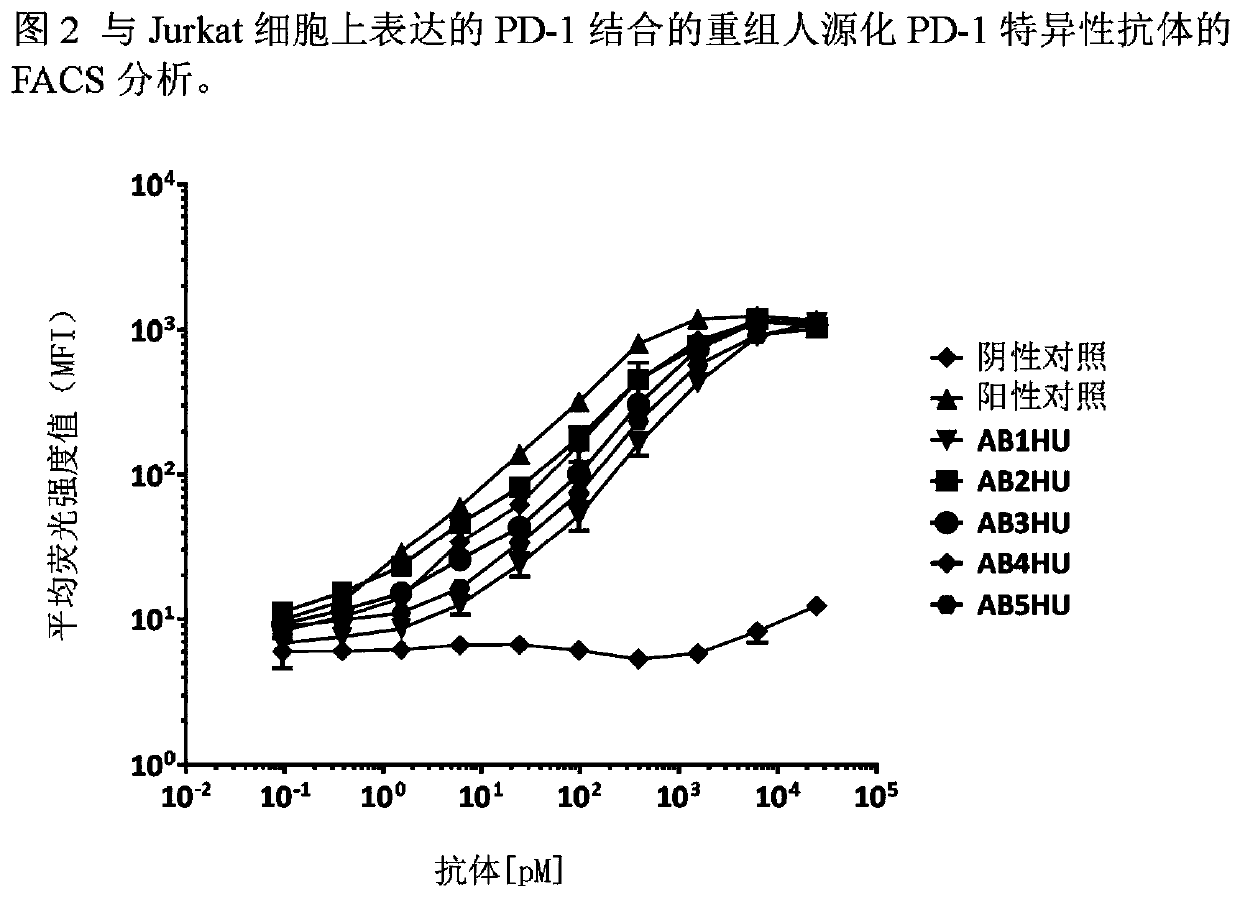
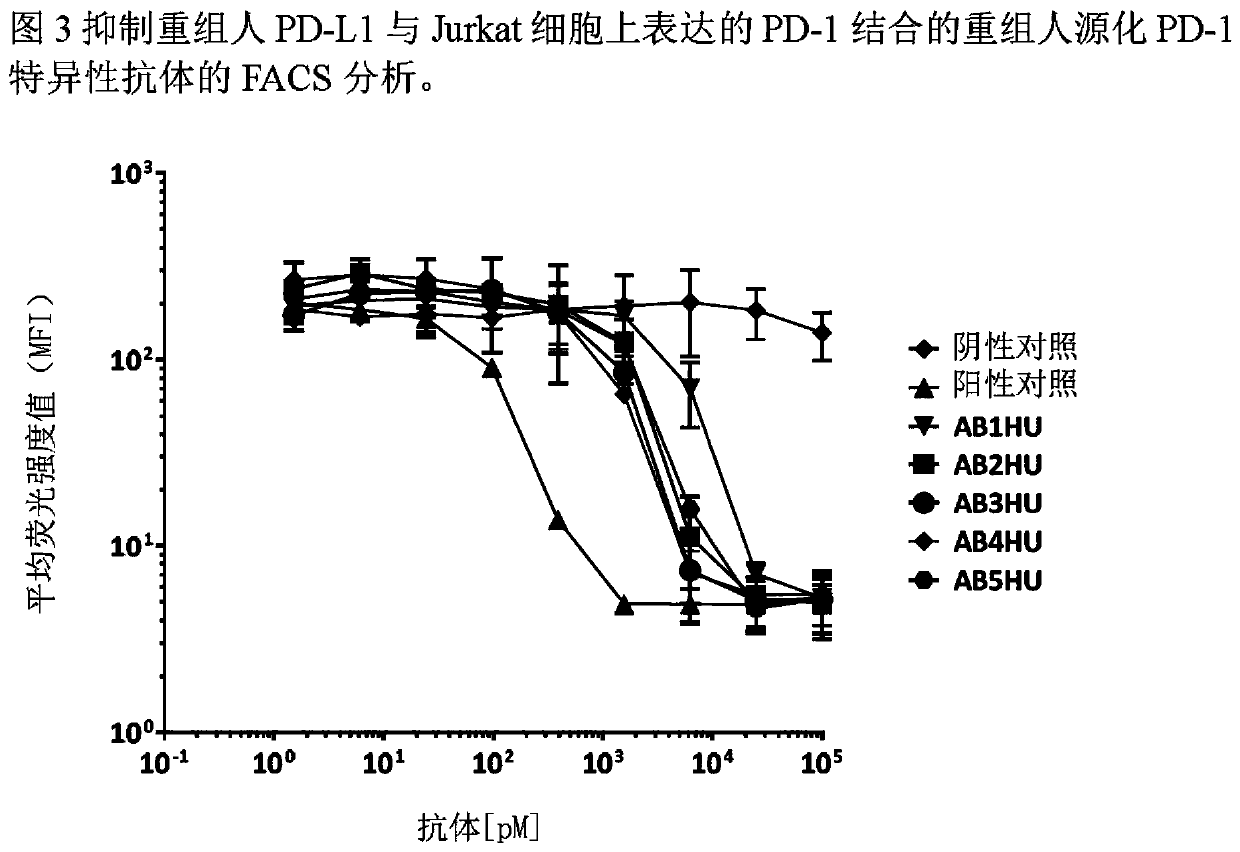
[0107] Example 3: Antibodies that bind to PD-1 expressed on the cell surface
[0108] The human PD-1 gene was subcloned into the pcDNA3.1 mammalian expression vector and transfected into the Jurkat human T cell line. Clonal cell populations expressing high levels of huPD-1 were generated by G418 drug selection and single cell sorting. The Jurkat / huPD-1 clonal cell line was labeled with FVS520 viability dye (BD Biosciences, diluted 1:2000) for 15 minutes at room temperature. Labeled cells were then diluted in FACS buffer (2% FBS in PBS), plated into V-bottom 96-well plates (50,000 cells per well) and incubated with 0-25 nM anti-huPDL1 or isotype control antibody Incubate on ice for 30 minutes. Cells were washed to remove excess primary antibody and then labeled with Alexa Luor647-conjugated goat anti-human Fc secondary antibody (Jackson ImmunoResearch, 1:1600 dilution) for 20 minutes on ice. Labeled cells were then washed with FACS buffer before harvesting on a BDFACSCalib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com