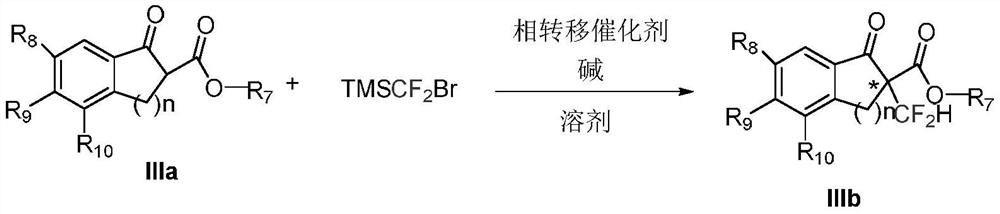
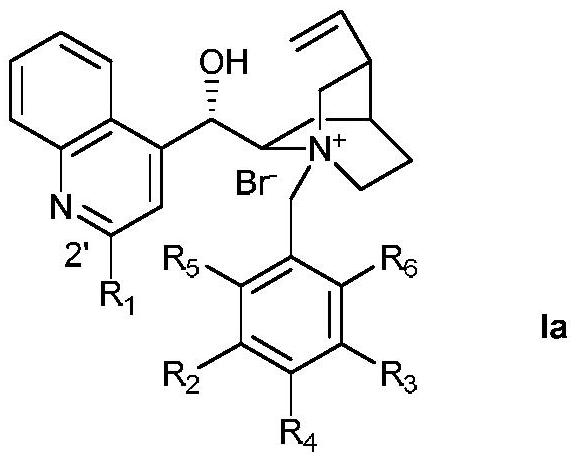
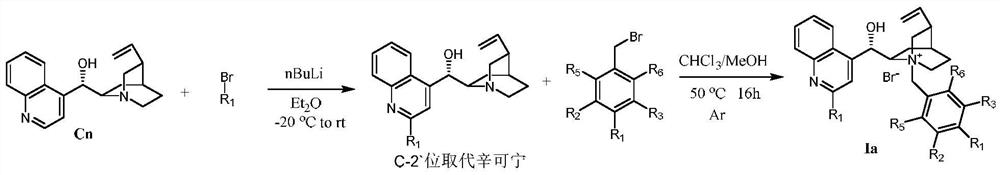
Phase transfer catalyzed asymmetric α-difluoromethylation of β-keto esters
A technology of phase transfer catalysis and difluoromethylation, applied in organic chemistry methods, chemical instruments and methods, catalytic reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Ia-1 (Ia, R 1 for H, R 2 -R 5 for H)
[0026]
[0027] 1.47 g of cinchonine, 50 mL of tetrahydrofuran, and 1.27 g of benzyl bromide were added to a 50 mL three-necked flask, and the mixture was refluxed for 16 hours under the protection of argon. After the reaction was completed, it was cooled to room temperature, the mixture was poured into 50 mL of ether, filtered, and the solid was recrystallized from methanol / ether to obtain Ia-1 with a yield of 88%.
Embodiment 2
[0029] Preparation of Ia-2 (Ia, R 1 is 4-trifluoromethylphenyl, R 2 -R 5 for H)
[0030]
[0031] Weigh 5.88 g (10 mmol) of cinchonine, add it into 75 mL of dry methyl tert-butyl ether solution, and protect it under argon. 15 mL of methyl tert-butyl ether was added dropwise to 4-bromobenzotrifluoride (11.26 g, 50 mmol) in methyl tert-butyl ether solvent at -20 °C to prepare 4-trifluoromethane by lithium-halogen exchange reaction phenyllithium reagent. The prepared organolithium reagent was quickly added to the cinchonine solution and stirred at -20°C for 1 hour. The mixture was then warmed to room temperature and stirring was continued for 2 hours. After the reaction, the system was quenched with 30 mL of acetic acid, and 60 mL of water and 60 mL of ethyl acetate were added. Then add 5 g of solid iodine and stir vigorously to dissolve it. Subsequently, 2 g of sodium thiosulfate dissolved in 20 mL of water was added to quench the iodine. The mixed solution was adjust...
Embodiment 3
[0033] Preparation of Ia-3 (Ia, R 1 is 4-trifluoromethylphenyl, R 2 -R 3 for CF 3 , R 4 -R 6 for H)
[0034]
[0035] Add 0.43 g of Cn', 8 mL of chloroform, 4 mL of methanol, and 0.46 g of 3,5-trifluoromethylbenzyl bromide into a 50 mL three-necked flask. The reaction was heated to 50°C for 16 hours. After the reaction was completed, it was cooled to room temperature, poured into 50 mL of diethyl ether, and filtered. The crude product was separated by column chromatography with dichloromethane / methanol=15:1, and then recrystallized with methanol / diethyl ether to obtain Ia-3 with a yield of 53%. 1 H NMR (400MHz, CD 3 OD)δ8.56(d,J=1.6Hz,2H),8.49–8.33(m,4H),8.22(s,1H),8.16–8.09(m,1H),7.89–7.71(m,4H), 6.63(d,J=2.5Hz,1H),6.09(ddd,J=17.4,10.4,7.2Hz,1H),5.59–5.44(m,1H),5.43–5.22(m,3H),4.56(ddd, J=11.7,8.4,2.7Hz,1H),4.17(dt,J=9.9,5.3Hz,2H),3.69–3.47(m,1H),3.13(dt,J=11.6,9.1Hz,1H),2.76 –2.41 (m, 2H), 2.03 – 1.76 (m, 3H), 1.15 – 1.02 (m, 1H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap