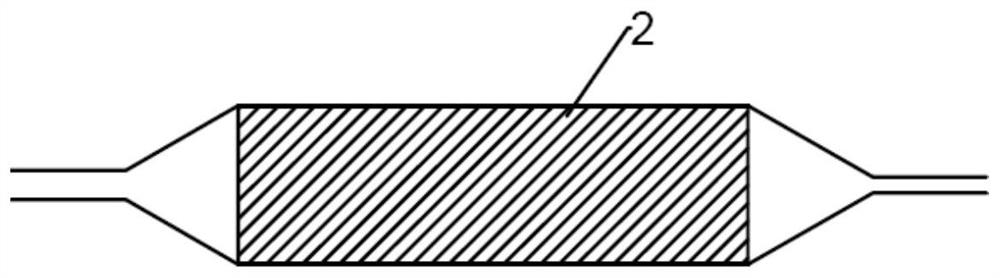
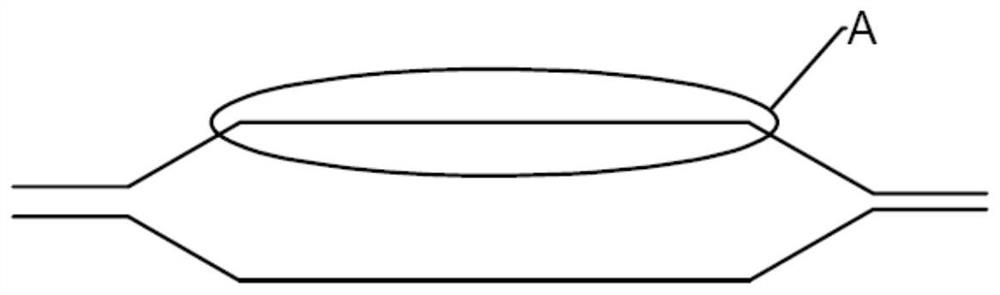
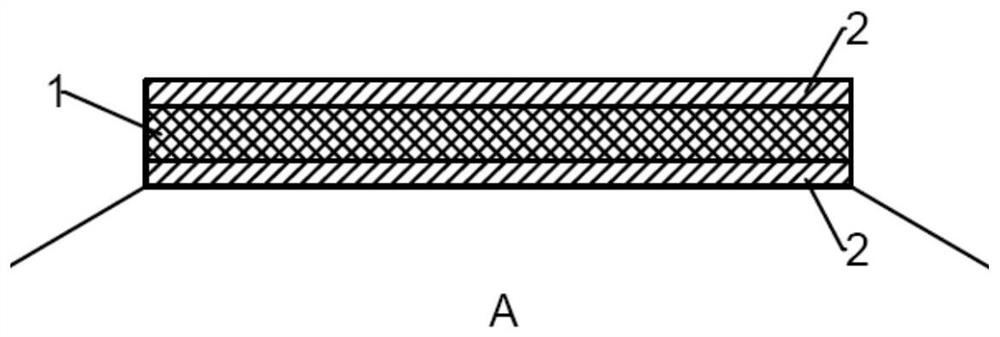
A drug balloon catheter and its preparation method
A balloon catheter and drug technology, applied in the direction of balloon catheters, catheters, drug devices, etc., can solve the problems of tissue necrosis, waste, poisoning of downstream tissues, etc., to reduce toxic side effects, improve utilization rate, and reduce drug loss Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Raw materials:
[0046] 1. Drug: Paclitaxel, Yunnan Hande Biotechnology Co., Ltd.;
[0047] 2. Carrier: PEG1000, Dow Chemical;
[0048] 3. Medical grade ethanol, Shanghai Aladdin Biochemical Technology Co., Ltd.
[0049] 2. Preparation method
[0050] S1, preparing a low-drug content coating solution, weighing 100 mg of paclitaxel and 100 mg of PEG1000 respectively, and dissolving them in 10 ml of medical grade ethanol to obtain a low-drug content coating solution, wherein the ratio of the drug to the mass of the solute is 50%.
[0051] S2, preparing a coating solution with high drug content, weighing 160 mg of paclitaxel and 40 mg of PEG1000 respectively, and dissolving them in 10 ml of medical grade ethanol to obtain a coating solution with high drug content, wherein the ratio of drug to solute mass is 80%.
[0052] S3, by means of ultrasonic spraying, the coating solution with low drug content is first sprayed on the surface of the balloon, sprayed twice, and a...
Embodiment 2
[0057] 1. Raw materials:
[0058] 1. Drug: rapamycin, Fujian Kerui Pharmaceutical Co., Ltd.;
[0059] 2. Carrier: lecithin, Shanghai Aladdin Biochemical Technology Co., Ltd.;
[0060] 3. Medical grade ethanol, Shanghai Aladdin Biochemical Technology Co., Ltd.
[0061] 2. Preparation method
[0062] S1, prepare coating solution with low drug content, take by weighing 20mg rapamycin and lecithin of 180mg respectively, dissolve in the medical grade ethanol of 10ml, obtain the coating solution with low drug content, wherein the ratio of drug to solute mass is 10%.
[0063] S2, preparing a coating solution with high drug content, weighing 100 mg rapamycin and 100 mg lecithin respectively, dissolving them in 10 ml of medical grade ethanol to obtain a coating solution with high drug content, wherein the ratio of drug to solute mass 50%.
[0064] S3, by means of ultrasonic spraying, the coating solution with low drug content is firstly sprayed onto the proximal and distal areas o...
Embodiment 3
[0068] 1. Raw materials:
[0069] 1. Drug: Tacrolimus, Fujian Kerui Pharmaceutical Co., Ltd.;
[0070] 2. Carrier: polyvinylpyrrolidone, Shanghai Aladdin Biochemical Technology Co., Ltd.;
[0071] 3. Medical grade ethanol, Shanghai Aladdin Biochemical Technology Co., Ltd.
[0072] 2. Preparation method
[0073] S1, preparing a low-drug content coating solution, weighing 120 mg of tacrolimus and 80 mg of polyvinylpyrrolidone, respectively, and dissolving them in 10 ml of medical grade ethanol to obtain a low-drug content coating solution, wherein the ratio of drug to solute mass 60%.
[0074] S2, preparing a coating solution with high drug content, weighing 200 mg of tacrolimus and dissolving it in 10 ml of medical grade ethanol to obtain a coating solution with low drug content, wherein the ratio of drug to solute mass is 100%.
[0075] S3, using the method of ultrasonic spraying, spraying the coating solution with low drug content on the surface of the balloon, spraying i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com