Prosthetic valves having a modified surface
A prosthetic valve, valve technology, applied in heart valve, prosthesis, anticoagulation treatment, etc., can solve the problem of controversial treatment selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
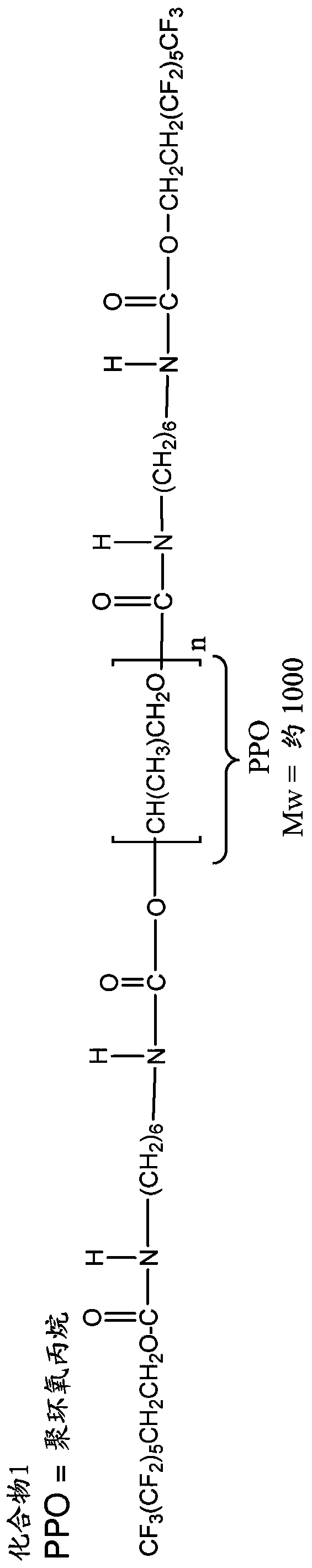
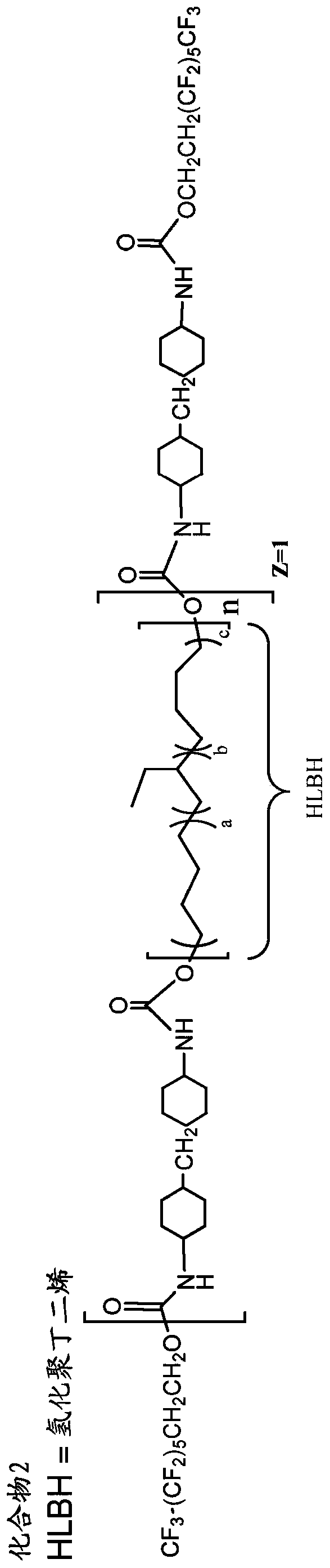
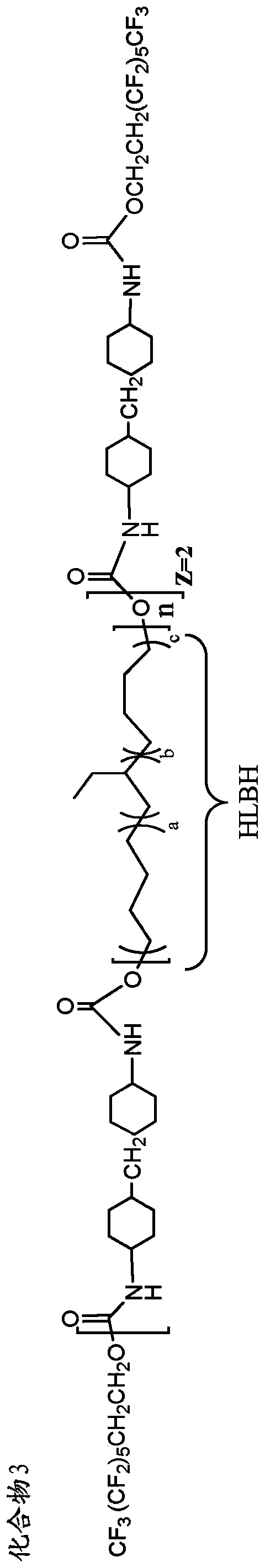
[0230] Example 1. Preparation of oligomeric fluorinated additives
[0231] The oligomeric fluorinated additives used in the prosthetic valves of the present invention can be prepared from appropriately selected reagents, such as diisocyanates / triisocyanates, dicarboxylic acids, diols, and fluorinated alcohols, using methods known in the art to form Wide range of oligomeric fluorinated additives. The reagents include, but are not limited to, the component reagents mentioned below.
[0232] Diisocyanate
[0233] HMDI=4,4'-methylene bis(cyclohexyl isocyanate)
[0234] IPDI = isophorone diisocyanate
[0235] TMXDI = meta-tetramethylene xylene diisocyanate
[0236] HDI = hexamethylene diisocyanate
[0237] Triisocyanate
[0238] Desmodur N3200 or Desmodur N-3200 = hexamethylene diisocyanate (HDI) biuret trimer
[0239] Desmodur Z4470A or Desmodur Z-4470A = isophorone diisocyanate (IPDI) trimer
[0240] Desmodur N3300 = hexamethylene diisocyanate (HDI) trimer
[0241] Diols...
Embodiment 2
[0355] Example 2. Preparation of Prosthetic Valves with Modified Surfaces
[0356] surface casting
[0357] The prosthetic valve according to the invention can be cast from a liquid mixture which is used to coat the structural support in the form of the valve or its components. In one embodiment, the liquid mixture is obtained by mixing, for example, dimethylacetamide (DMAc), tetrahydrofuran (THF), isopropanol (IPA) and oligomeric fluorinated additives (such as formulas (I)-(XVII) A solution of any one of the compounds or any one of Compounds 1-41; a target dry weight percentage of the oligomeric fluorinated additive in the final coating is 0.05% by weight to 15% by weight) with a suitable base polymer (eg Bionate TM 、Elast-Eon TM , 2363-80AE Elastomer, SIBS, xSIBS, BIOSPAN TM or ELASTHANE TM ) solution was prepared by mixing. The bowl was then fitted into a planetary mixer with paddle blades and the contents were stirred at room temperature for 30 minutes. The coatin...
Embodiment 3
[0362] Example 3. BCA analysis of protein deposition
[0363] Reference prosthetic valves of the invention were prepared (eg, as described in Example 2) and incubated in varying concentrations of protein solutions. Examples of proteins that can be used in this assay include fibrinogen, albumin and lysozyme. The concentration of protein is typically in the range of 1 mg / mL to 5 mg / mL. The incubation time is typically about 2 hours to about 3 hours. After the incubation was complete, film samples were rinsed with PBS. Adhesion of proteins to the sample can then be quantified using methods known in the art, eg, bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL). Briefly, the samples were incubated in a solution of sodium dodecyl sulfate (SDS) solution for up to about 24 hours (under sonication if necessary) to remove the protein from the surface. A working solution was then prepared using the kit, which facilitates the reduction of copper ions and interaction with the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com