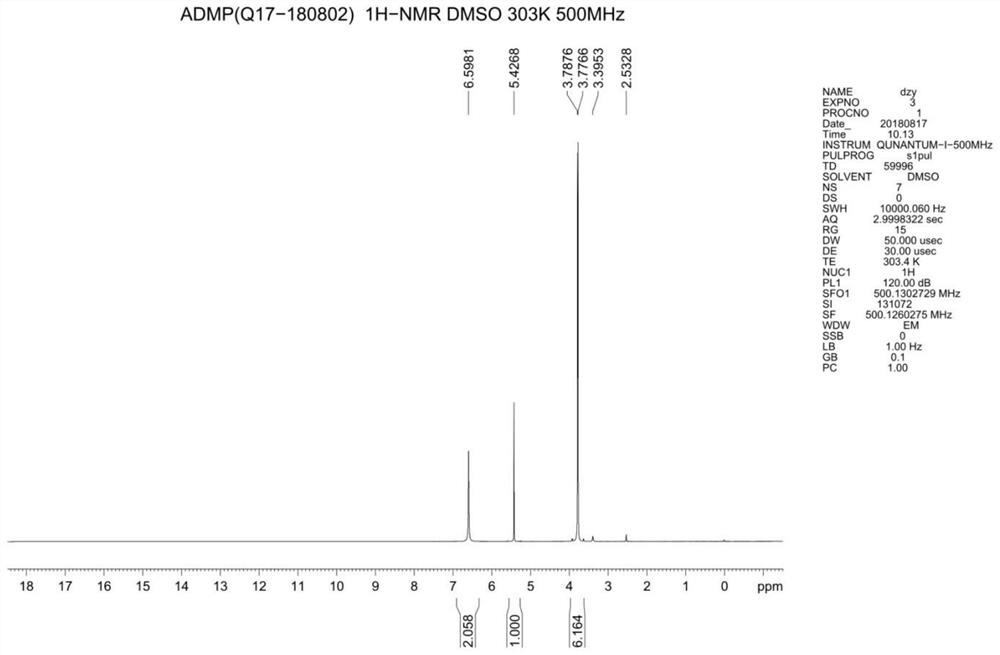
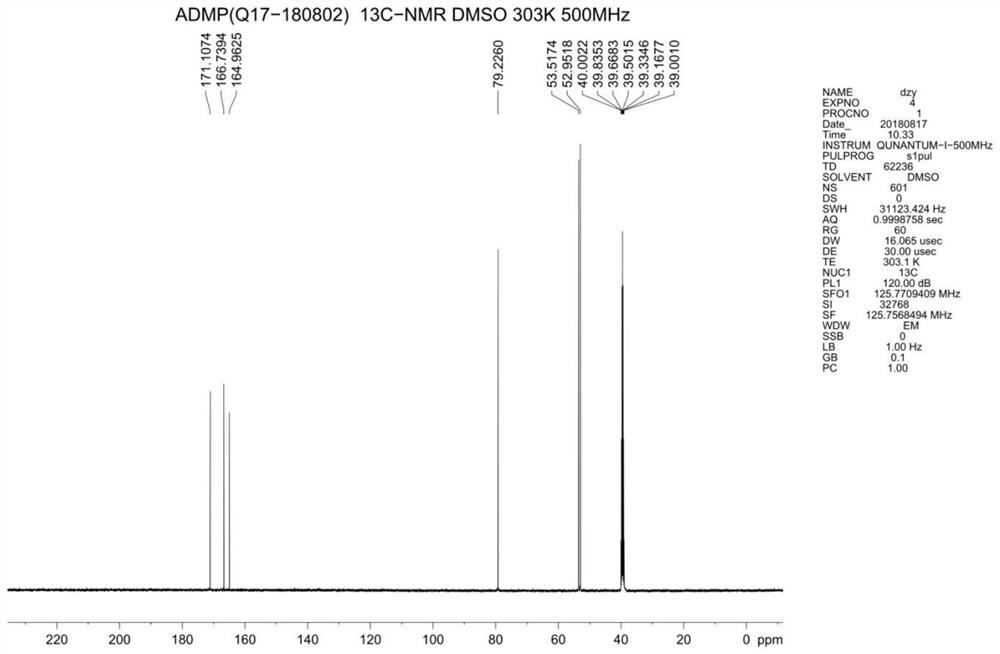
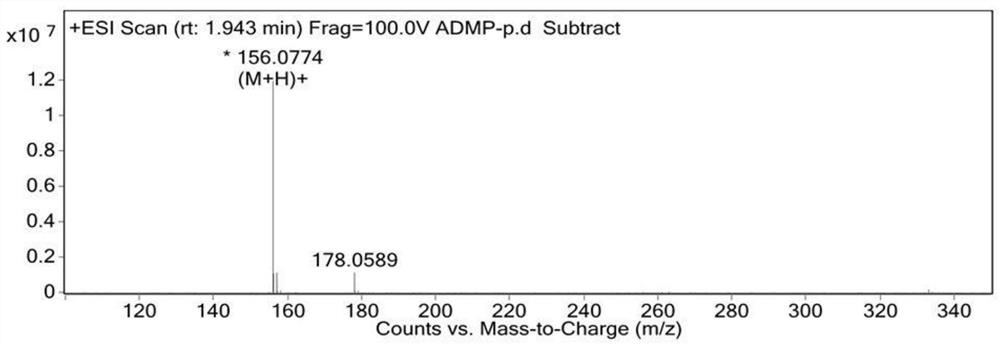
A kind of synthetic method of 4-amino-2.6-dimethoxypyrimidine
A technology of dimethoxypyrimidine and a synthesis method, which is applied in the field of synthesis of 4-amino-2.6-dimethoxypyrimidine, can solve the problems of increased process risk, low yield in the chlorination step, a large amount of phosphorus-containing waste water and the like , to avoid emissions, reduce environmental pollution, and save costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprising the steps:
[0032] (1) 300ml of water, 26.5g of 28% ammonia water were added in a 500mL four-necked reaction flask, 40g of 4,6-dichloropyrimidine-5-carboxylic acid was added under stirring, and then the temperature was kept at 30°C and reacted for 6 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0033] (2) after the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-carboxylic acid to 3 with hydrochloric acid, feed about 15g of chlorine, and the logical chlorine process control temperature is between 10~40 ℃, and the logical chlorine finishes, Adjust pH to 4~5 with liquid alkali, filter below 20℃ to obtain 4-amino-2,6-dichloropyrimidine-5-carboxylic acid tidal product;
[0034] (3) add 1000ml water in 2L pressure reaction kettle, drop into the 4-amino-2,6-dichloropyrimidine-5-carboxylic acid obtained in step (2), be warming up to 150 ℃, react for 2 hours, complete the rea...
Embodiment 2
[0037] A synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprising the steps:
[0038] (1) 300ml of water, 32.7g of 28% ammonia water were added to the 500mL four-necked reaction flask, 40g of 4,6-dichloropyrimidine-5-carboxylic acid was added under stirring, and then the temperature was kept at 50°C and reacted for 12 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0039] (2) after the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-carboxylic acid to 3 with hydrochloric acid, feed about 15g of chlorine, and the logical chlorine process control temperature is between 10~40 ℃, and the logical chlorine finishes, Adjust pH to 4~5 with liquid alkali, filter below 20℃ to obtain 4-amino-2,6-dichloropyrimidine-5-carboxylic acid tidal product;
[0040] (3) add 1000ml water in 2L pressure reactor, drop into the 4-amino-2,6-dichloropyrimidine-5-carboxylic acid wet product that step (2) obtains, be warming up to 110 ℃, react 4 hours, reaction fin...
Embodiment 3
[0043] A synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprising the steps:
[0044] (1) 300ml of water, 32.7g of 28% ammonia water were added to the 500mL four-necked reaction flask, 40g of 4,6-dichloropyrimidine-5-carboxylic acid was added under stirring, and then the temperature was kept at 50°C and reacted for 12 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0045](2) after the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-carboxylic acid to 3 with hydrochloric acid, feed about 15g of chlorine, and the logical chlorine process control temperature is between 10~40 ℃, and the logical chlorine finishes, Adjust pH to 4~5 with liquid alkali, filter below 20℃ to obtain 4-amino-2,6-dichloropyrimidine-5-carboxylic acid tidal product;
[0046] (3) add 1000ml water in 2L pressure reactor, drop into the 4-amino-2,6-dichloropyrimidine-5-carboxylic acid wet product that step (2) obtains, be warming up to 110 ℃, react 4 hours, reaction fini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com