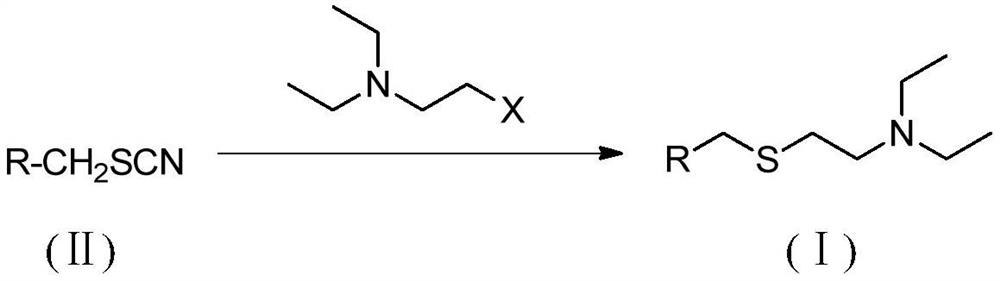
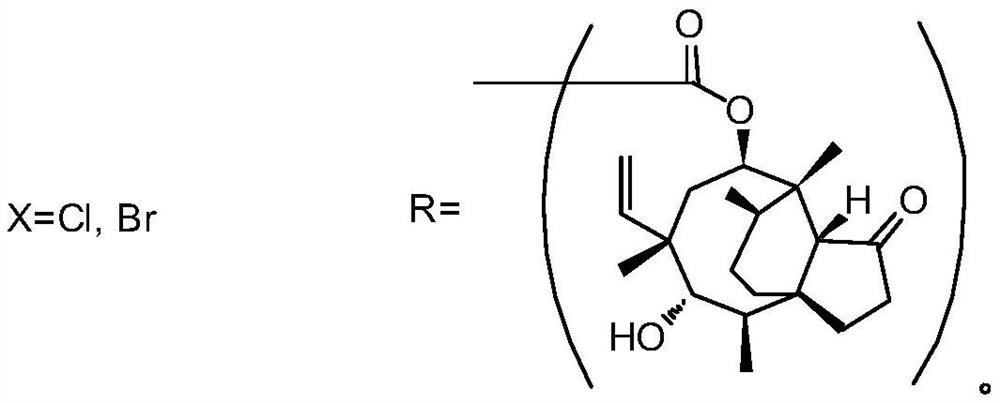
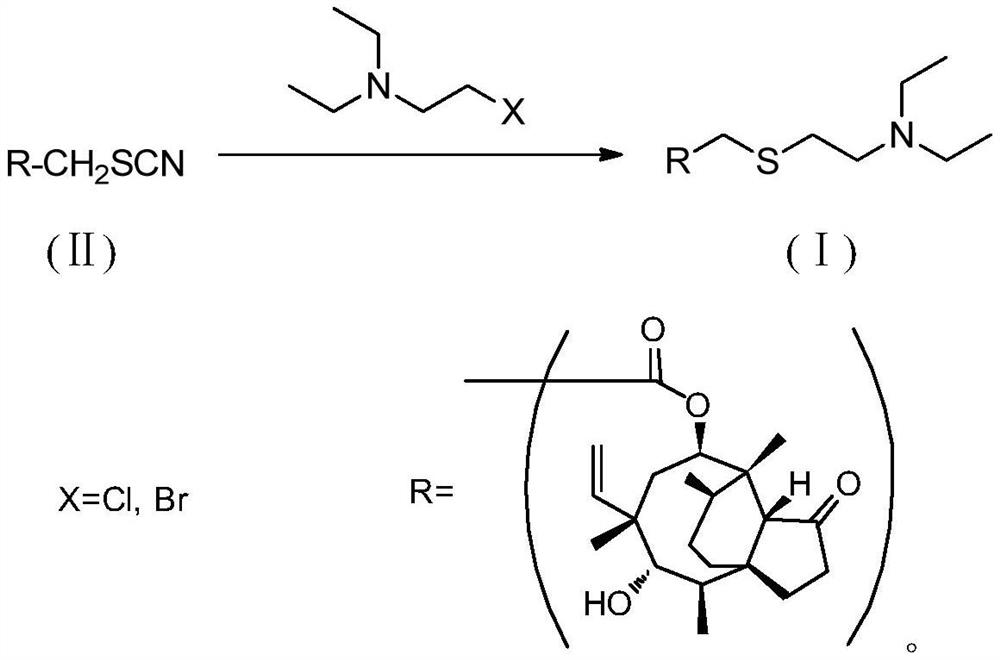
A method for preparing tiamectin
A technology of tiamectin and tiamectin thiocyanate, which is applied in the field of preparation of veterinary antibiotics, can solve the problems of restricting product market scale and cost control, difficulty in refining and removing, safety impact, etc., and achieves easy operation and market promotion, improve market competitiveness, and reduce the effect of control costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 tiamectin (Ⅰ)
[0025] Add tiamectin thiocyanate (II) (41.9g, 0.1mol), acetone (830g) and 20% aqueous solution of sodium sulfide nonahydrate (145g) in the reactor, stir at room temperature for 30 minutes, mix well and then cool to 10-15°C, keep it for 30 minutes, and then add a solution of 2-bromo-N,N-diethylethylamine (21.6g, 0.12mol) and acetone (85g). The addition process does not exceed 10 minutes. After the completion, remove the cooling device, naturally rise to room temperature and continue to stir for 4 hours. After the reaction is completed, pour the reaction mixture into a large amount of water, use 5N hydrochloric acid solution to adjust the pH to 8, continue stirring for 30 minutes, collect the precipitated solid by filtration, and dry Finally, a white or light yellow solid powder was obtained, which was 47.2 g of crude tiamectin (I) with a purity of 98.5% and a yield of 95.7%. After recrystallization from toluene, 45.4 g of r...
Embodiment 2
[0027] The preparation of embodiment 2 tiamectin (Ⅰ)
[0028] Add tiamectin thiocyanate (II) (41.9g, 0.1mol), acetone (505g) and 20% aqueous solution of sodium sulfide nonahydrate (130g) in the reactor, stir at room temperature for 30 minutes, mix well and then cool to 10-15°C, keep it for 30 minutes, and then add a solution of 2-bromo-N,N-diethylethylamine (19.8g, 0.11mol) and acetone (85g). The addition process does not exceed 10 minutes. After completion, remove the cooling device, naturally rise to room temperature and continue to stir the reaction for 0.5 hours. After the reaction is completed, pour the reaction mixture into a large amount of water, use 5N hydrochloric acid solution to adjust the pH to 8, continue stirring for 30 minutes, collect the precipitated solid by filtration, and dry Finally, a white or light yellow solid powder was obtained, which was 43.1 g of crude tiamectin (I) with a purity of 98.1% and a yield of 87.4%. After recrystallization from toluene, ...
Embodiment 3
[0030] The preparation of embodiment 3 tiamectin (Ⅰ)
[0031] Add tiamectin thiocyanate (II) (41.9g, 0.1mol), acetone (650g) and 20% aqueous sodium sulfide nonahydrate (138g) into the reactor, stir at room temperature for 30 minutes, mix well and then cool to 10-15°C, keep it for 30 minutes, and then add a solution of 2-bromo-N,N-diethylethylamine (20.7g, 0.115mol) and acetone (85g). The addition process does not exceed 10 minutes. After completion, remove the cooling device, naturally rise to room temperature and continue stirring for 2 hours. After the reaction, pour the reaction mixture into a large amount of water, adjust the pH to 8 with 5N hydrochloric acid solution, continue stirring for 30 minutes, collect the precipitated solid by filtration, and dry Finally, a white or light yellow solid powder was obtained, which was 45.1 g of crude tiamectin (I) with a purity of 98.5% and a yield of 91.5%. After recrystallization from toluene, 42.8 g of fine tiamectin (I) was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com