Novel chlorphenamine maleate oxidation impurity and preparation process thereof
A technology for oxidizing impurities and chlorpheniramine, which is applied in organic chemistry and other fields, can solve the problems of difficult separation and purity, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
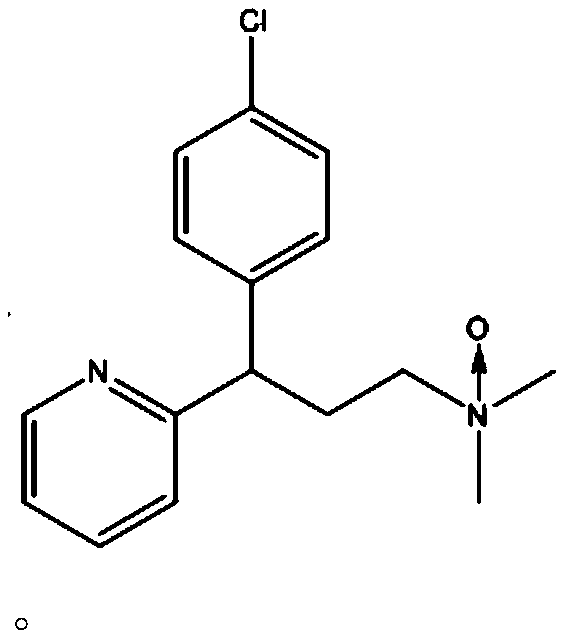
[0032] Embodiment 1, the preparation of chlorpheniramine oxidation impurity of the present invention
[0033]
[0034] Add 20g of chlorpheniramine and 200ml of dichloromethane into a 500ml single-necked bottle, stir to dissolve, cool down to -10°C, add 14.8g of sodium bicarbonate, add 30.4g of m-chloroperoxybenzoic acid (m-CPBA) in batches, and React at -10°C for 2 hours, TLC monitors the completion of the reaction, add 200ml of water, stir and separate layers, take the organic layer and add 100ml of saturated brine to wash, take the organic layer and add anhydrous sodium sulfate to dry to obtain a crude product. Then take the crude product and pass it through a silica gel column (200-300 mesh silica gel, column length 20cm, eluent is dichloromethane:methanol=10:1v / v) to obtain 15g of the target product (i.e. the oxidized impurity of chlorpheniramine of the present invention). Yield 72.37%, purity 97%.
[0035] Structural characterization of the target product: MS(m / z): 29...
experiment example 1
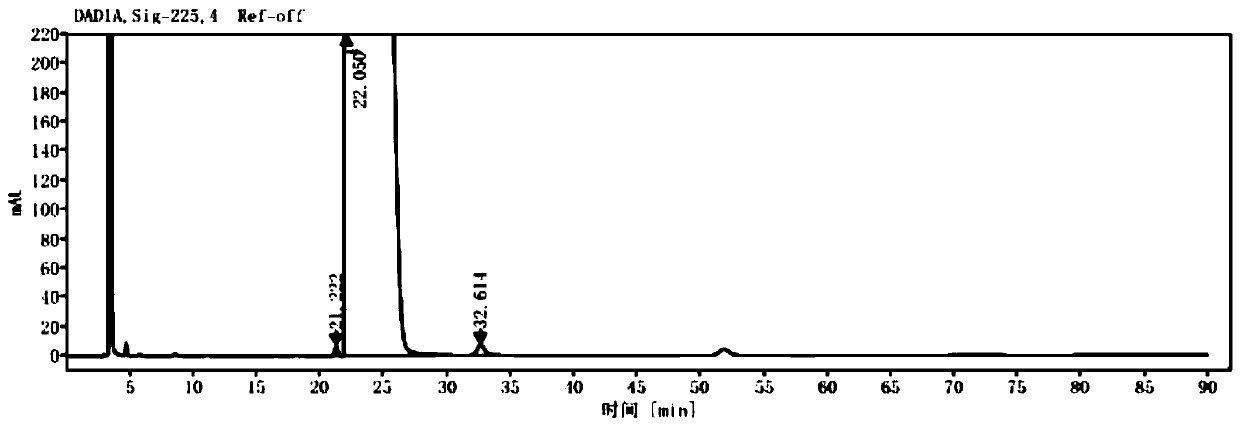
[0037] Experimental example 1, the effect of chlorpheniramine oxidized impurities of the present invention in the quality detection of chlorpheniramine test product as positive reference substance
[0038] 1. Experimental method
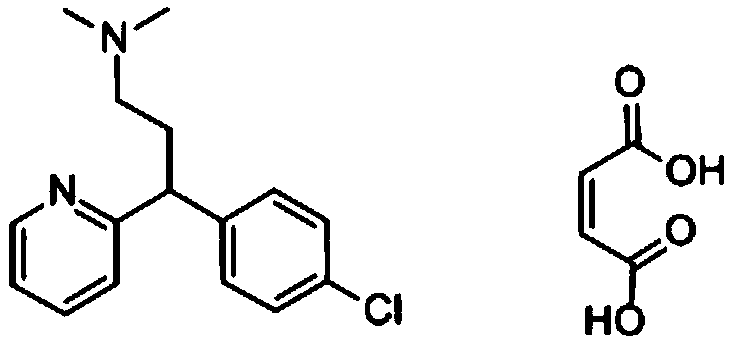
[0039] According to the high performance liquid phase method (general rule 0512), measure the content of chlorpheniramine maleate oxidation impurity in the chlorpheniramine maleate test solution. Among them, chlorpheniramine maleate is a commercially available product or prepared according to a known method (such as the method described in patent application 201910251936.4).
[0040] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler, and the chromatographic column was Ultimate XDB-C18 (4.6*300mm, 5μm); 8.57g / L NH 4 h 2 PO 4 (adjust pH 3.0 with phosphoric acid)-acetonitrile (80:20) as mobile phase; detection wavelength 225nm; flow rate 1.0ml / min; column temperature 35°C. Take 20ul of the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com