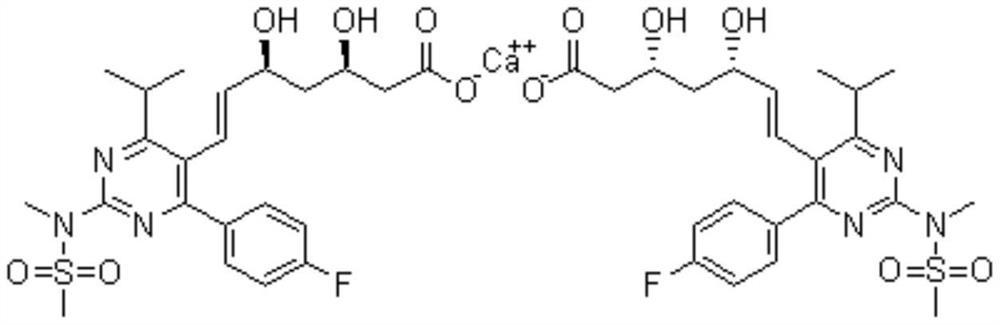
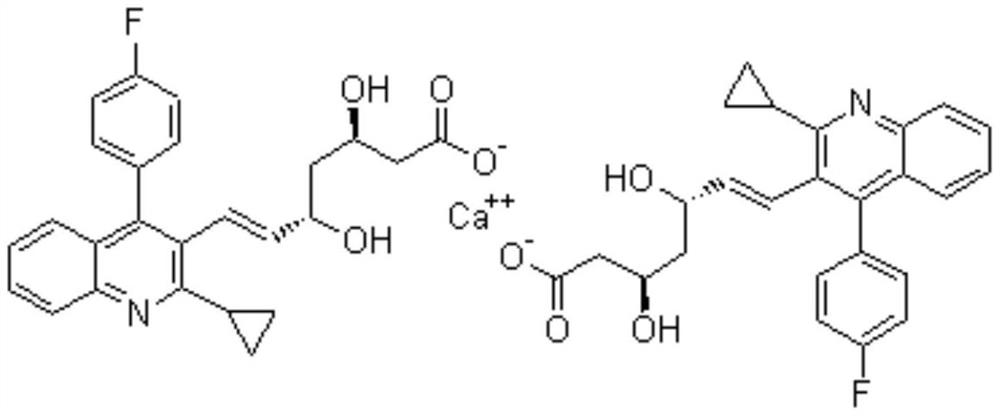
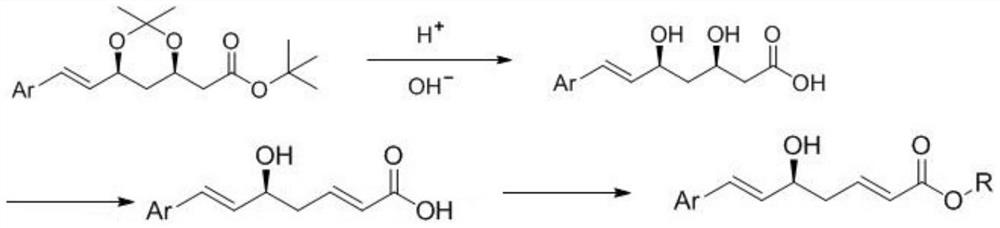
A method for preparing rosustatin and pitavastatin 2,6-diene enanthate compound
A technology of diene enanthate and pitavastatin, which is applied in the field of preparation of rosustatin and pitavastatin 2,6-diene enanthate compound, can solve the problem of expanding the application of limited drugs, complex process routes, Low preparation yield and other problems, to achieve the effect of short and easy synthetic route, high product yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of rosuvastatin acid:
[0029]
[0030] In a 1000mL reaction flask, add (4R,6S)-6-[(1E)-2-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino ]-5-pyrimidine]vinyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester (45g, 78mmol, 1.0eq) and 400mL tetrahydrofuran, stirred and dissolved, cooled to At 5-10°C, acetic acid (4.7g, 78mmol, 1.0eq) was slowly added dropwise. After dropping, the temperature was raised to 30-35°C for reaction, and the reaction was monitored by TLC. After the reaction was completed, the temperature was lowered to 5-10° C., and 98 mL of 2N lithium hydroxide solution (195 mmol, 2.5 eq) was added dropwise. After dropping, the temperature was raised to 30-35°C for reaction, and the reaction was monitored by TLC. After the reaction was completed, the temperature was lowered to room temperature, and the pH was adjusted to nearly neutral with 1N hydrochloric acid solution. Part of the solvent was distilled off under reduc...
Embodiment 2
[0035] Preparation of rosuvastatin 2,6-diene heptanoate compound:
[0036] 1. Preparation of rosuvastatin 2,6-diene heptanoic acid intermediate:
[0037] In a 500mL reaction flask, add rosuvastatin acid (30g, 63mmol, 1.0eq) and 300mL tetrahydrofuran, stir to dissolve, cool down to 5-10°C, slowly add sulfuric acid (12.3g, 126mmol, 2.0eq) dropwise under nitrogen protection . After dropping, the temperature was raised to room temperature for reaction, and the reaction was monitored by TLC. After the reaction is complete, pour the reaction solution into 600 mL of ice water, stir, add sodium hydroxide (7.5 g, 189 mmol, 3.0 eq), stir for 10 min, react with diethyl ether (300 mL×1), discard the organic layer, and wash the aqueous layer with 2N Adjust the pH to neutral with sodium hydroxide solution, extract with ethyl acetate (200mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate under reduced pressure to obtain 2...
Embodiment 3
[0049] Preparation of pitavastatin 2,6-diene heptanoate compound:
[0050] 1. Preparation of pitavastatin 2,6-diene heptanoic acid intermediate:
[0051] In a 1000mL reaction flask, add pitavastatin acid (45g, 107mmol, 1.0eq) and 400mL tetrahydrofuran, stir to dissolve, cool to 5-10°C, add potassium tert-butoxide (24g, 214mmol, 2.0eq ). After dropping, the temperature was raised to room temperature for reaction, and the reaction was monitored by TLC. After the reaction was completed, the reaction solution was poured into 600 mL of ice water, stirred, reacted with diethyl ether (300 mL×1), the organic layer was discarded, the pH of the aqueous layer was adjusted to neutral with 2N hydrochloric acid solution, and extracted with ethyl acetate (200 mL×3) , the organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated under reduced pressure to obtain 35.3 g of pitavastatin 2,6-diene heptanoic acid intermediate prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com