A method for preparing ns5a inhibitor-velpatasvir
A technology of inhibitors and reagents, applied in the field of drug synthesis, can solve the problems of less than 50% total cure rate, unsuitable production and preparation, and limited drug application, etc., and achieve the effects of enhanced operability, easy handling and purification, and simplified preparation routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
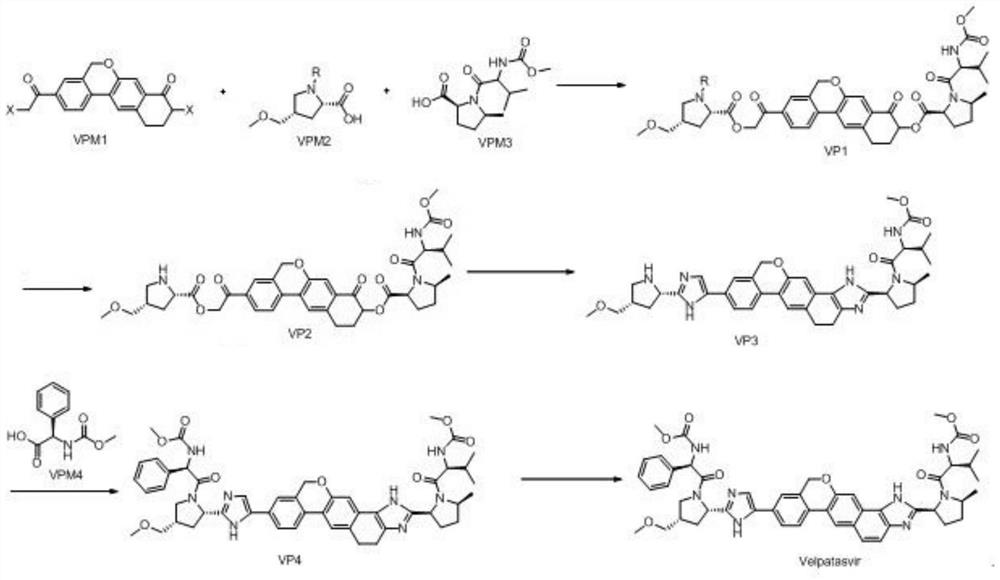
[0029] The synthetic route is as follows:
[0030]
[0031] Step 1, preparation of VP1:
[0032] At room temperature, add 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8 into a 1000mL reaction flask (9H)-Kone (VPM1) (20g, 45mmol, 1.0eq), (2S,4S)-1-(tert-butoxycarbonyl)-4-(methoxymethyl)-pyrrolidine-2-carboxylic acid ( VPM2) (11.6g, 46mmol, 1.02eq), cesium carbonate (29.3g, 90mmol, 2.0eq) and 300mL tetrahydrofuran were stirred, heated to 50-55°C for reaction, and monitored by TLC. When TLC showed that the reaction residue of raw material VPM2 was below 2%, it was lowered to 30-35° C., and then 100 mL of VPM3 in tetrahydrofuran (13.2 g, 46 mmol, 1.02 eq) was added dropwise. After dropping, the temperature was raised to 50-55°C for reaction, and the reaction was monitored by TLC. After the reaction is complete, cool to room temperature, add 500mL of water and 500mL of ethyl acetate, stir, let stand for liquid separation, extract the aqueous layer wi...
Embodiment 2
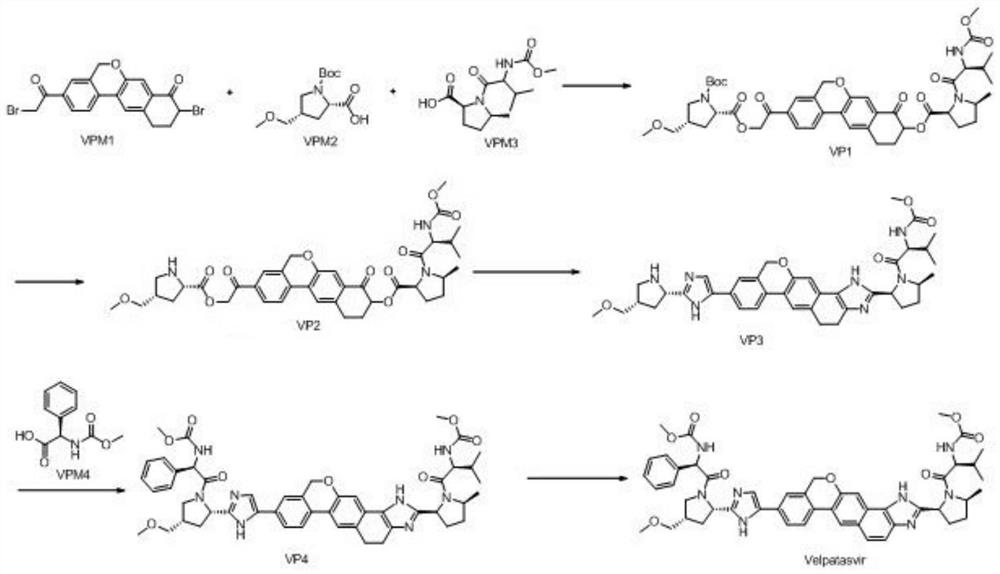
[0042] The synthetic route is as follows:
[0043]
[0044] Step 1, preparation of VP1:
[0045] At room temperature, add 9-bromo-3-(2-chloroacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran-8 into a 1000mL reaction flask (9H)-Kone (VPM1) (30g, 74mmol, 1.0eq), (2S,4S)-1-(benzyloxycarbonyl)-4-(methoxymethyl)-pyrrolidine-2-carboxylic acid (VPM2 ) (22.9g, 78mmol, 1.05eq), potassium carbonate (30.6g, 222mmol, 3.0eq) and 400mL of acetonitrile, stirred, heated to 50-55°C for reaction, TLC monitored the reaction. When TLC showed that the reaction residue of the raw material VPM2 was below 2%, it was lowered to 30-35° C., and then 100 mL of VPM3 in acetonitrile (22.3 g, 78 mmol, 1.05 eq) was added dropwise. After dropping, the temperature was raised to 50-55°C for reaction, and the reaction was monitored by TLC. After the reaction is complete, cool to room temperature, add 500mL of water and 600mL of ethyl acetate, stir, let stand for liquid separation, extract the aqueous la...
Embodiment 3
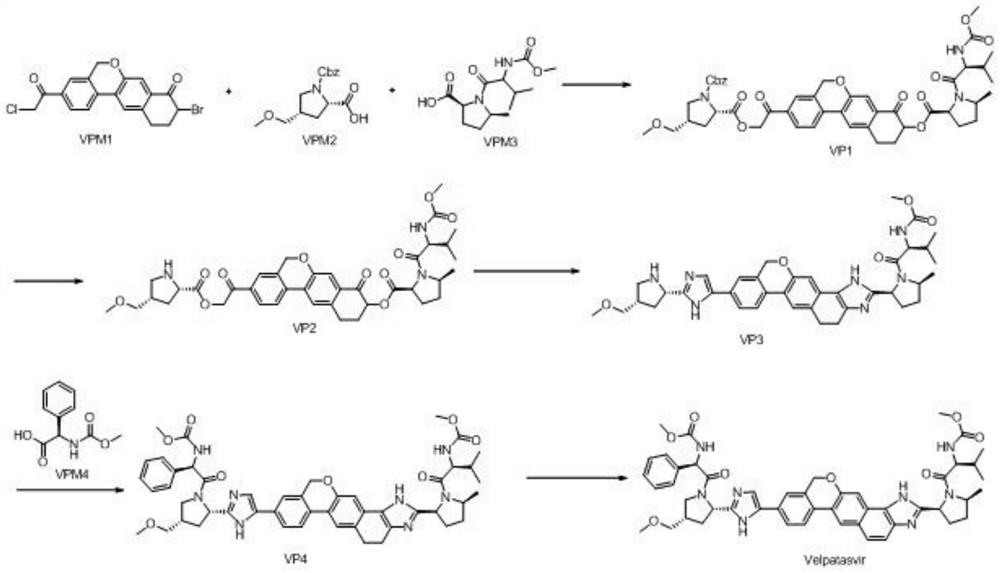
[0055] The synthetic route is as follows:
[0056]
[0057] Step 1, preparation of VP1:
[0058] At room temperature, in a 500mL reaction flask, 9-bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[D]naphtho[2,3-B]pyran -8(9H)-Kone (VPM1) (15g, 33mmol, 1.0eq), (2S,4S)-1-(benzoyl)-4-(methoxymethyl)-pyrrolidine-2-carboxylic acid (VPM2) (9.2g, 35mmol, 1.05eq), cesium carbonate (23.3g, 66mmol, 2.0eq) and 200mL tetrahydrofuran were stirred, heated to 50-55°C for reaction, and monitored by TLC. When TLC showed that the reaction residue of raw material VPM2 was below 2%, it was lowered to 30-35° C., and then 80 mL of VPM3 in tetrahydrofuran (10 g, 35 mmol, 1.05 eq) was added dropwise. After dropping, the temperature was raised to 50-55°C for reaction, and the reaction was monitored by TLC. After the reaction is complete, cool to room temperature, add 300mL of water and 400mL of ethyl acetate, stir, let stand for liquid separation, extract the aqueous layer with ethyl acetate (150mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com