Composition for diagnosing colorectal cancer and method for diagnosing colorectal cancer by using composition
A colorectal cancer and composition technology, applied in biochemical equipment and methods, disease diagnosis, biomaterial analysis, etc., can solve the problems of low sensitivity, compliance and low distribution rate, and achieve the effect of improving survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Serum Acquisition
[0064] Sera were obtained from 290 normal subjects (male: 176 cases, female: 114 cases) at the Department of Family Medicine, Seoul National University Hospital. Sera were obtained from 75 and 25 colorectal cancer patients (male: 46 cases, female: 54 cases) in the Biobank of Keimyung University Dongsan Medical Center and the Biobank of Pusan National University Hospital, respectively. Peripheral blood (5 ml) was collected from normal or colorectal cancer patients, put into Vacutainer SST II tubes (BD, USA), and placed at room temperature. After 1 hour, the tubes were centrifuged at 3000g for 5 minutes and the supernatant was removed to obtain serum. The obtained serum was stored at -80°C until use.
Embodiment 2
[0065] Example 2: Protein Quantification
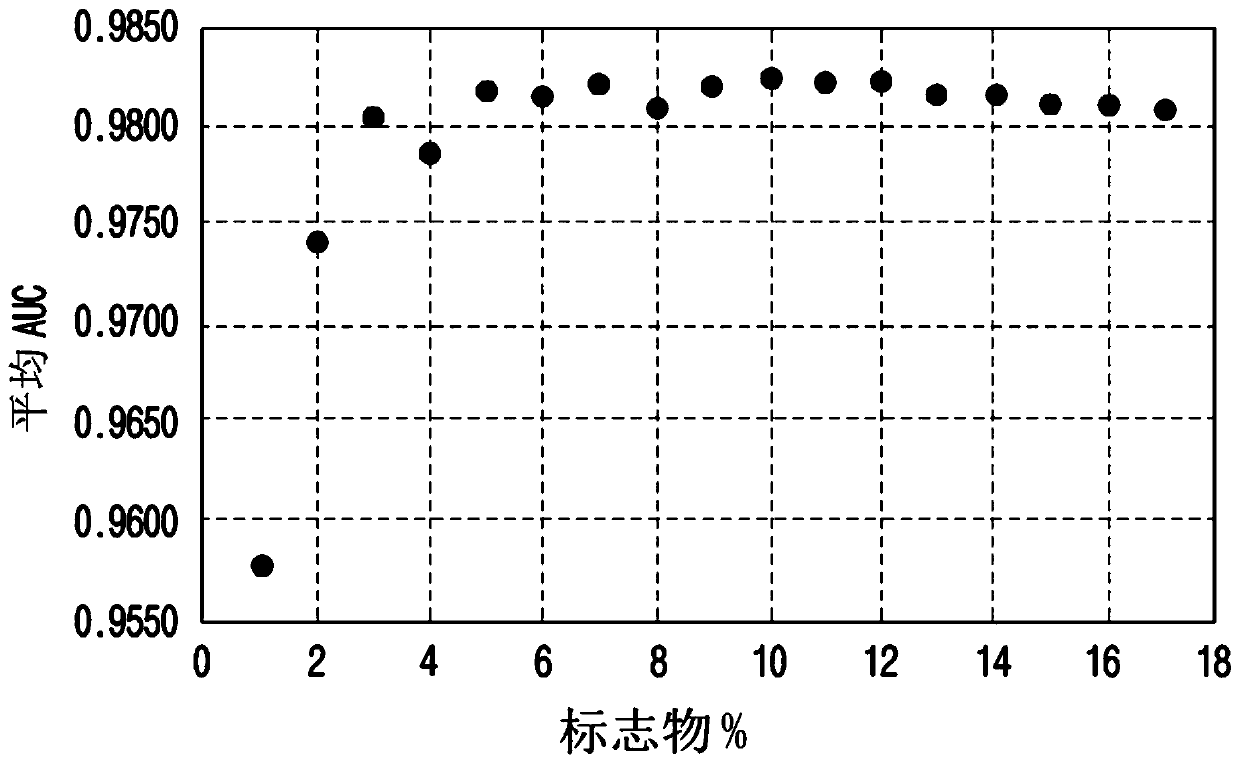
[0066] To screen colorectal cancer biomarker candidates, a panel of 120 protein marker candidates was first selected from thousands of proteins in the human body. Then, approximately 50 markers were selected from a candidate panel of 120 protein markers using 2D electrophoresis and SELDI-TOF MS, considering clinical significance, ease of analysis, algorithm accuracy, cost, and clinical condition. The curative effect was studied by statistical analysis of the results of more than 150 colorectal cancer patients and 400 normal subjects. Finally, 17 proteins were selected, such as ApoA1, ApoA2, AFP, CEA, CA125, CA19-9, B2M, CRP, CYFRA21-1, VDBP, PAI-1, sVCAM-1, RANTES, EGFR, ApoA4, TTR and D. Dimers As colorectal cancer biomarker candidates, 17 proteins were quantified using the serum samples of Example 1 to conduct their statistical analysis as follows.
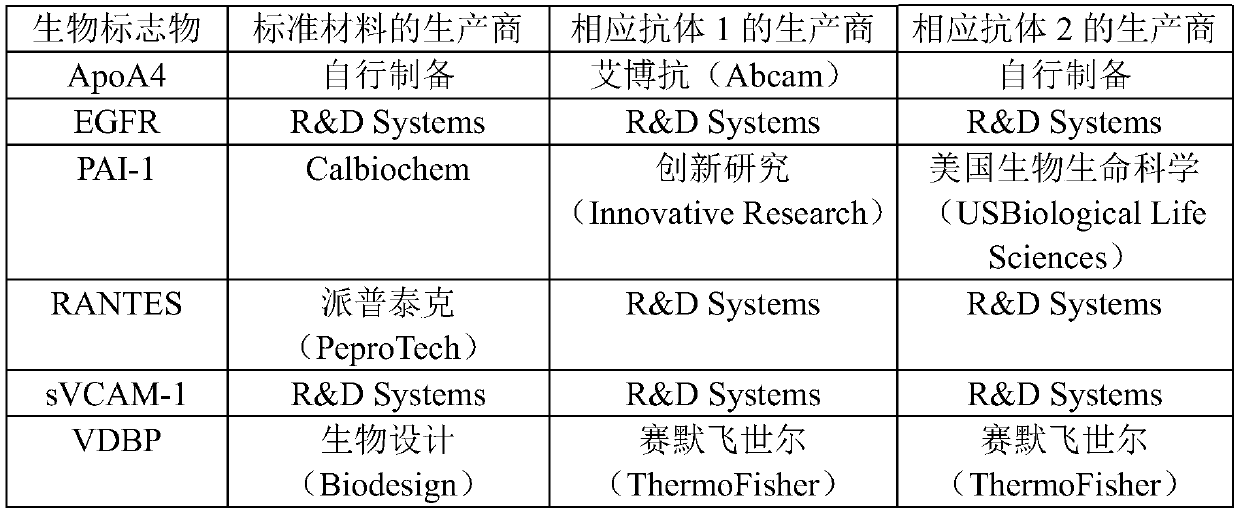
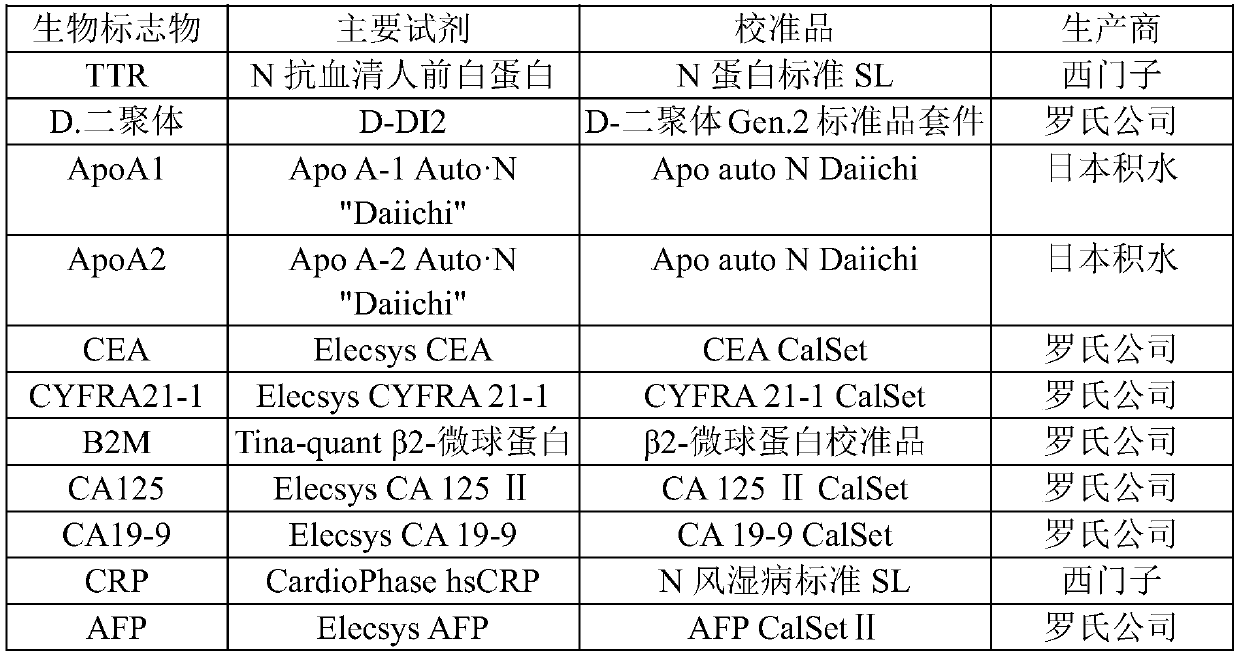
[0067] 2-1. Antibodies, kits and standard proteins
[0068] Quantification of Ap...
Embodiment 3
[0085] Example 3: Statistical Analysis of Colorectal Cancer Biomarkers
[0086] 17 markers (ApoA1, ApoA2, AFP, CEA, CA125, CA19-9, B2M, CRP, CYFRA21-1, VDBP, PAI-1, sVCAM-1 , RANTES, EGFR, ApoA4, TTR and D. dimer) the difference in the expression level was significant. The experimental value obtained in embodiment 2 is carried out Log 10 transformed, and these values (measured data) were used for statistical analysis. When the p value obtained by the t test was below 0.1, it was determined as a significant marker.
[0087] 【table 3】
[0088] Significance of markers
[0089]
[0090]
[0091] As shown in Table 3, ApoA1, ApoA2, AFP, CEA, CA125, CA19-9, B2M, CRP, CYFRA21-1, VDBP, PAI-1, sVCAM-1, RANTES , EGFR, ApoA4, TTR and D. There were significant differences in the expression levels of dimer markers (Table 3).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap