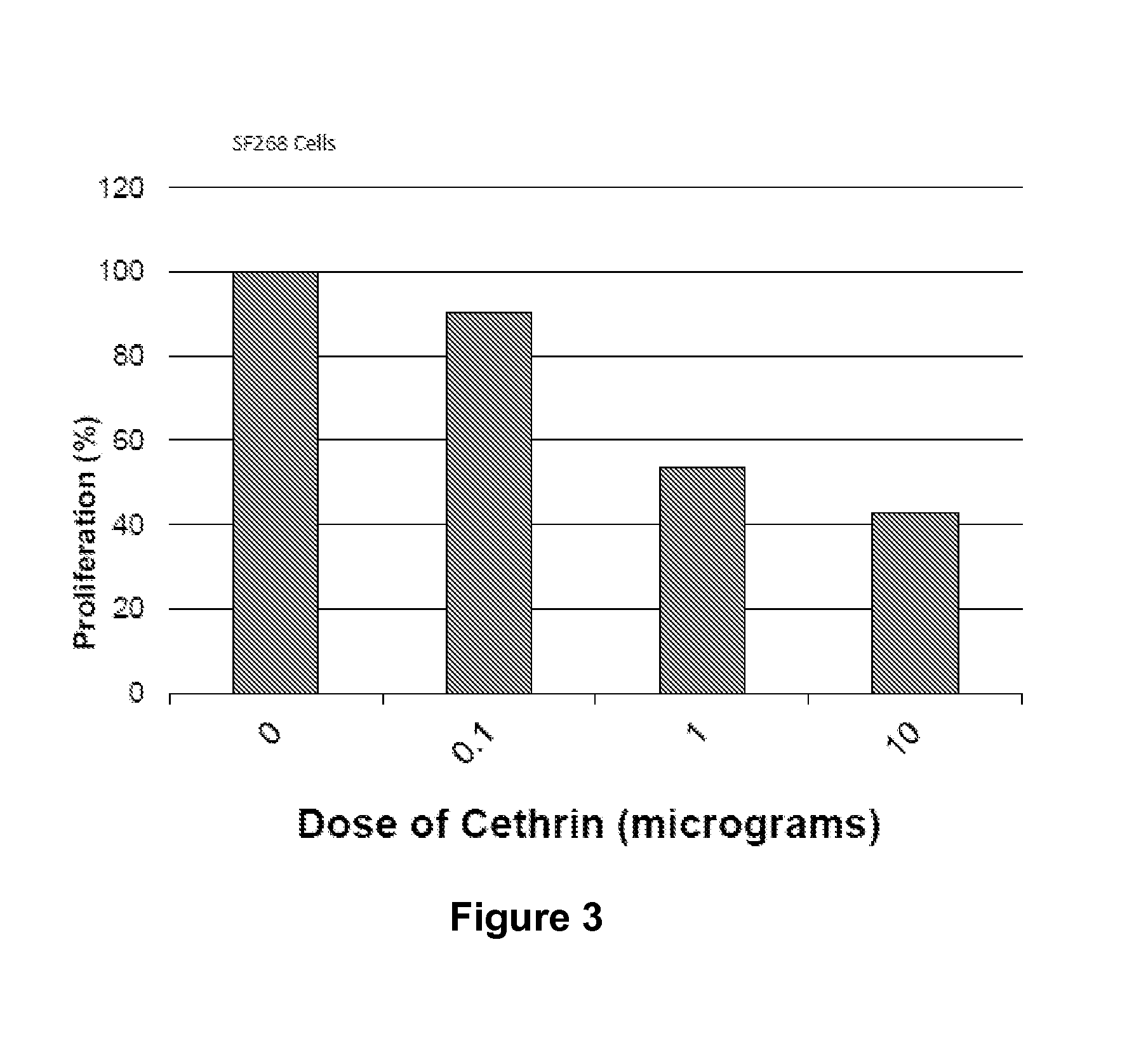
Patents
Literature
123 results about "Early Therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Procedures undertaken to prevent or reduce the incidence or progression of disease, in individuals with EARLY DIAGNOSIS of disease, or known DISEASE SUSCEPTIBILITY.
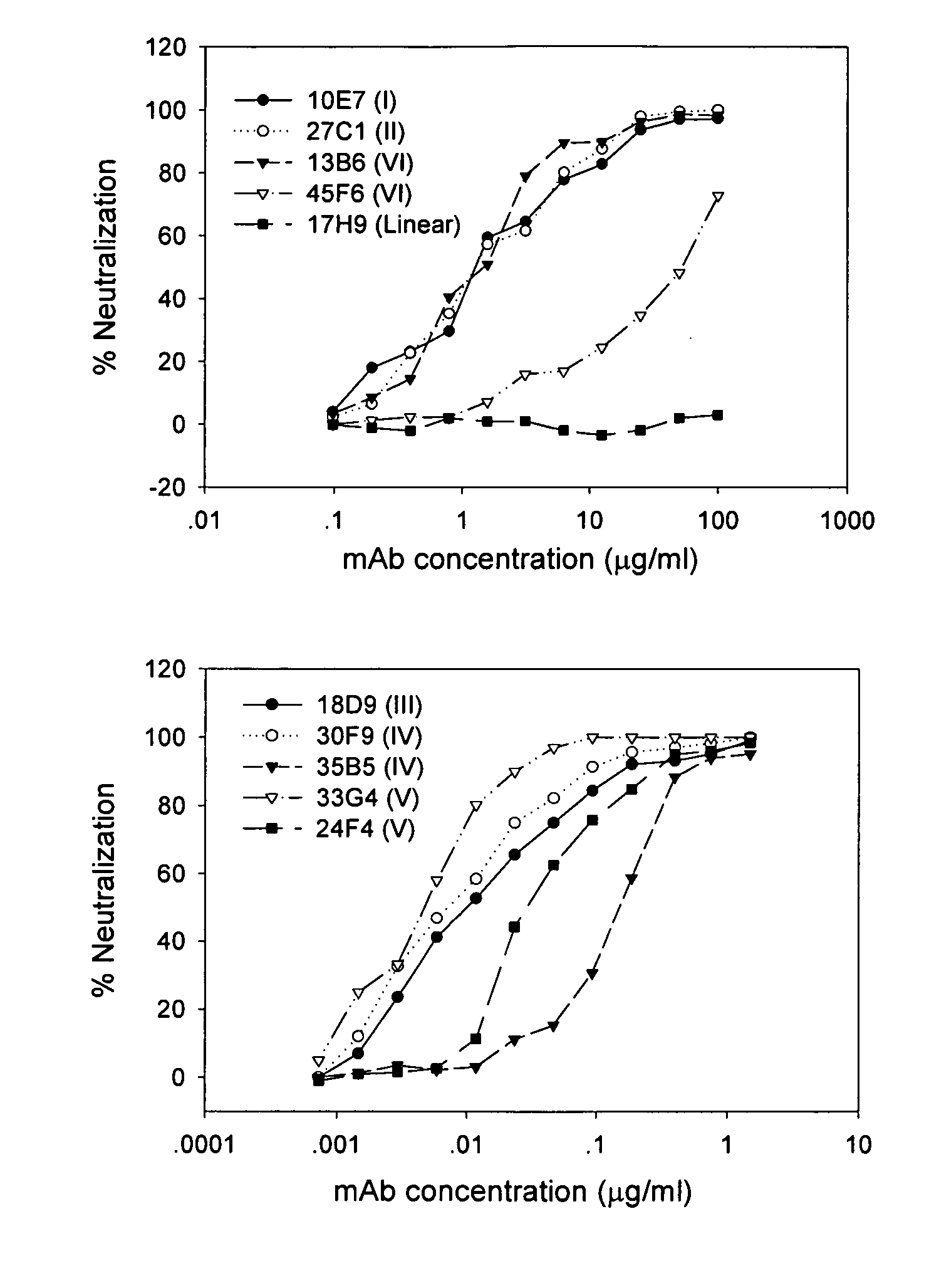
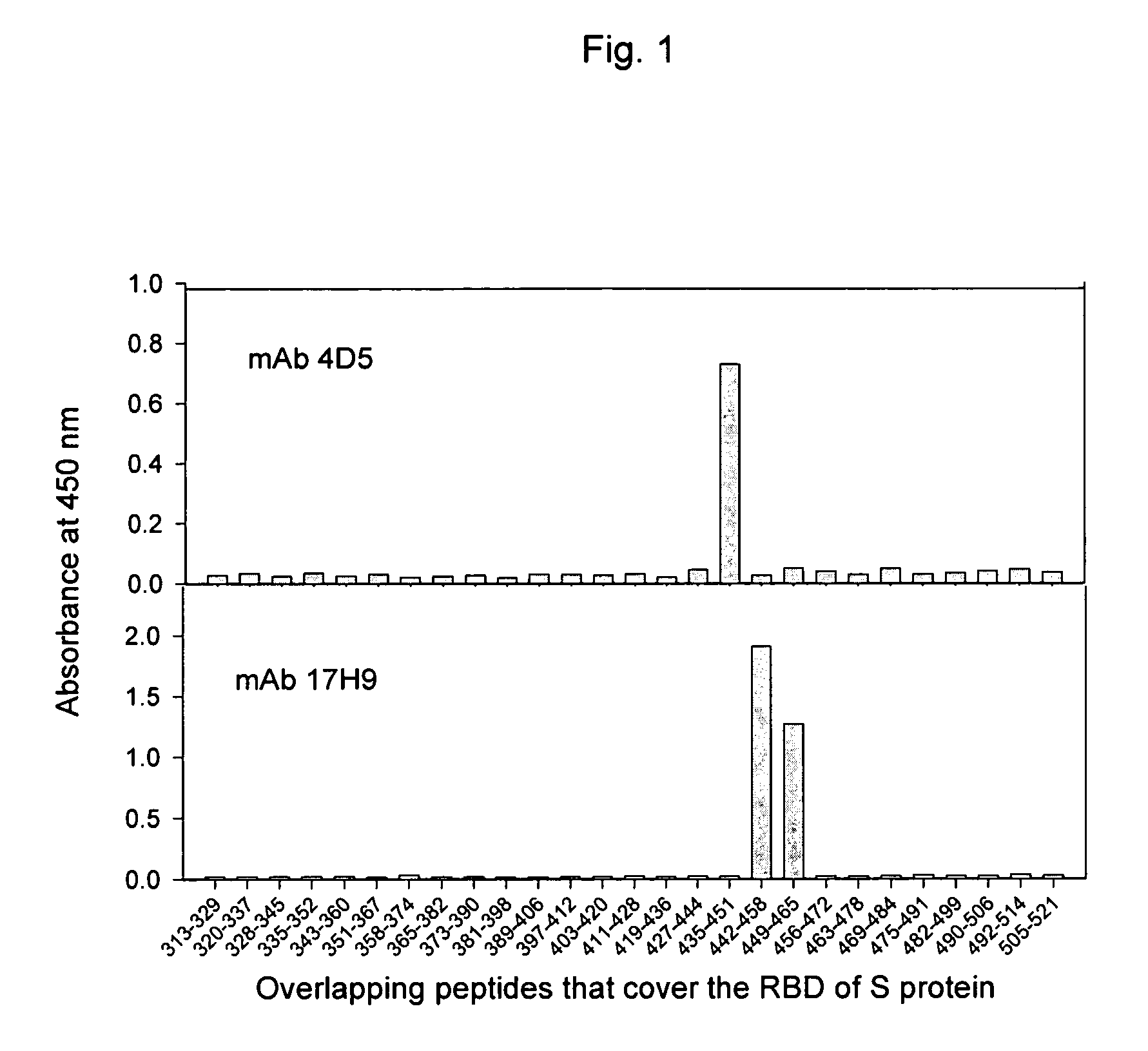
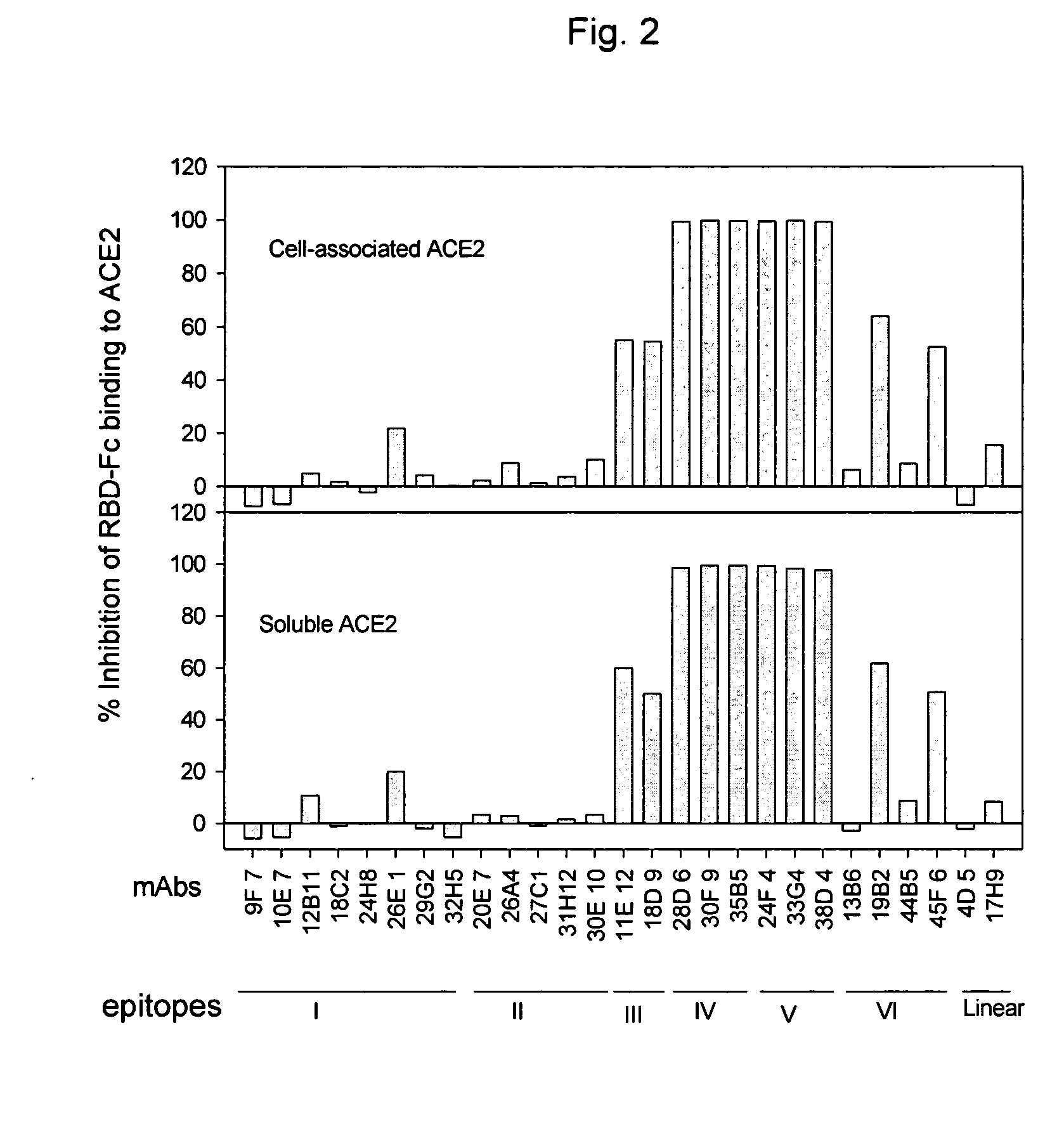
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus
InactiveUS20060240551A1Reduce the binding forceAvoid infectionAnimal cellsMicrobiological testing/measurementAntigenicitySpike Protein
The present invention provides an isolated antibody capable of binding to the receptor-binding domain of the spike protein of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) so as to competitively inhibit the binding of the SARS-CoV to host cells. These mAbs or substances can be used: 1) as passive-immunizing agents for prevention of SARS-CoV infection; 2) as biological reagents for diagnosis of SARS-CoV infection; 3) as immunotherapeutics for early treatment of SARS-CoV infection; and 4) as probes for studying the immunogenicity, antigenicity, structure, and function of the SARS-CoV S protein.
Owner:NEW YORK BLOOD CENT
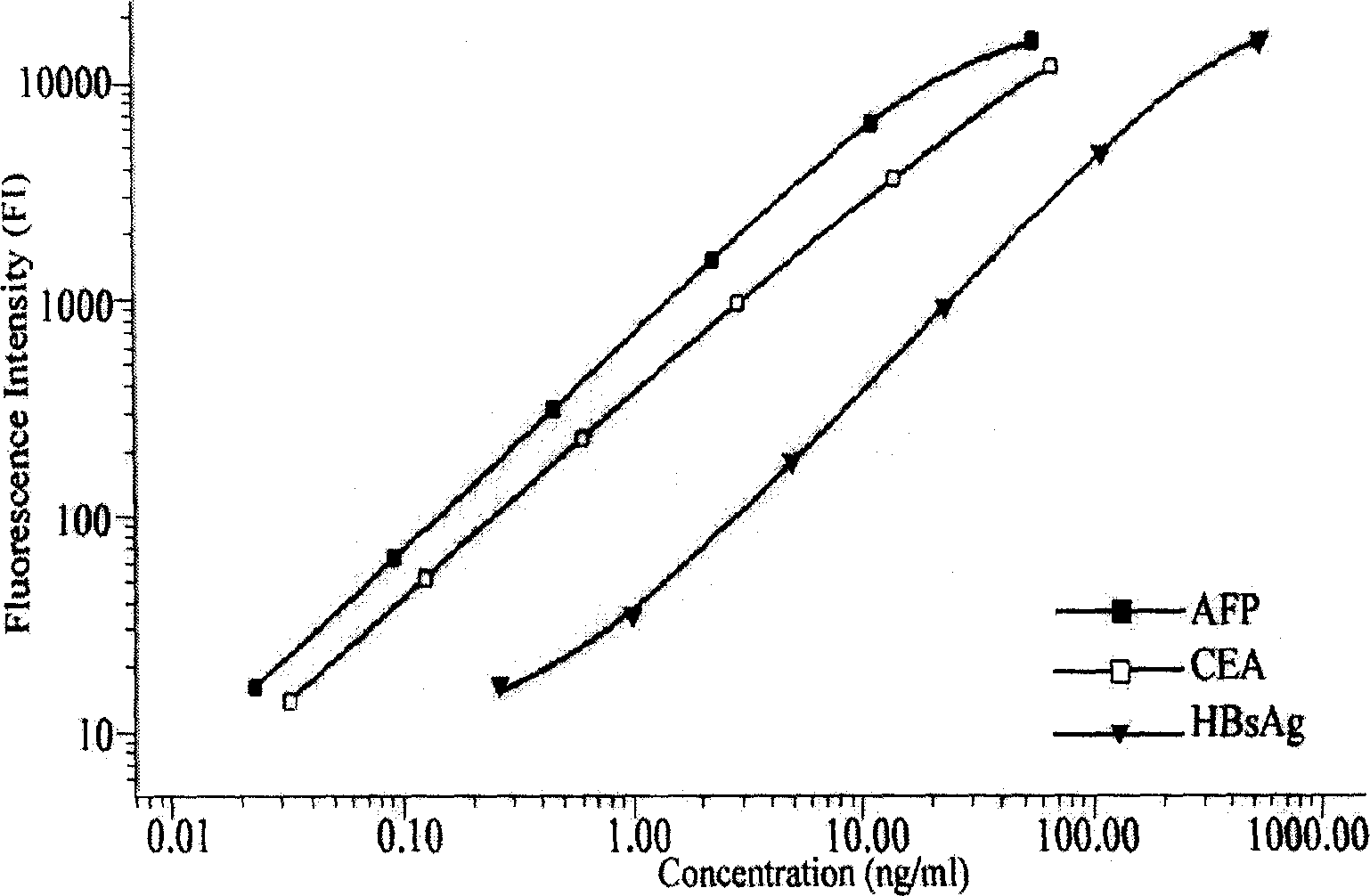
Preparation method of liquid phase protein chip
InactiveCN101144815AEarly detectionEarly treatmentMaterial analysisHigh risk populationsFluorescence
The present invention relates to the biologic technology field, and discloses a liquid phase albumen chip and the preparation and the usage method thereof which can simultaneously test human serum carcinoma embryonic antigen (CEA), Alpha fetoprotein (AFP), and hepatitis B surface antigen (HBsAg). The present invention couples the specificity antibody of CEA, AFP, and HBsAg on different fluorescence micro-spheres, and uses the test antibody marked by biotin or phycoerythrin to determine the nature quickly and quantitatively analyze the above three indexes with the double antibody sandwich method. The present invention uses the filtering membrane board when testing, and washes the board 3 times after each reaction is finished to increase the signal and improve the sensitivity. The present invention has high sensitivity, strong specificity, stable result, excellent repeatability, and simple operation; 1 micro liter serum sample can test three indexed simultaneously. The present invention is applicable to the health test and the general examination as well as the clinic test of the high risk population, and can facilitate the early diagnosis and the early treatment of the knub.
Owner:GUANGZHOU DARUI BIOTECH
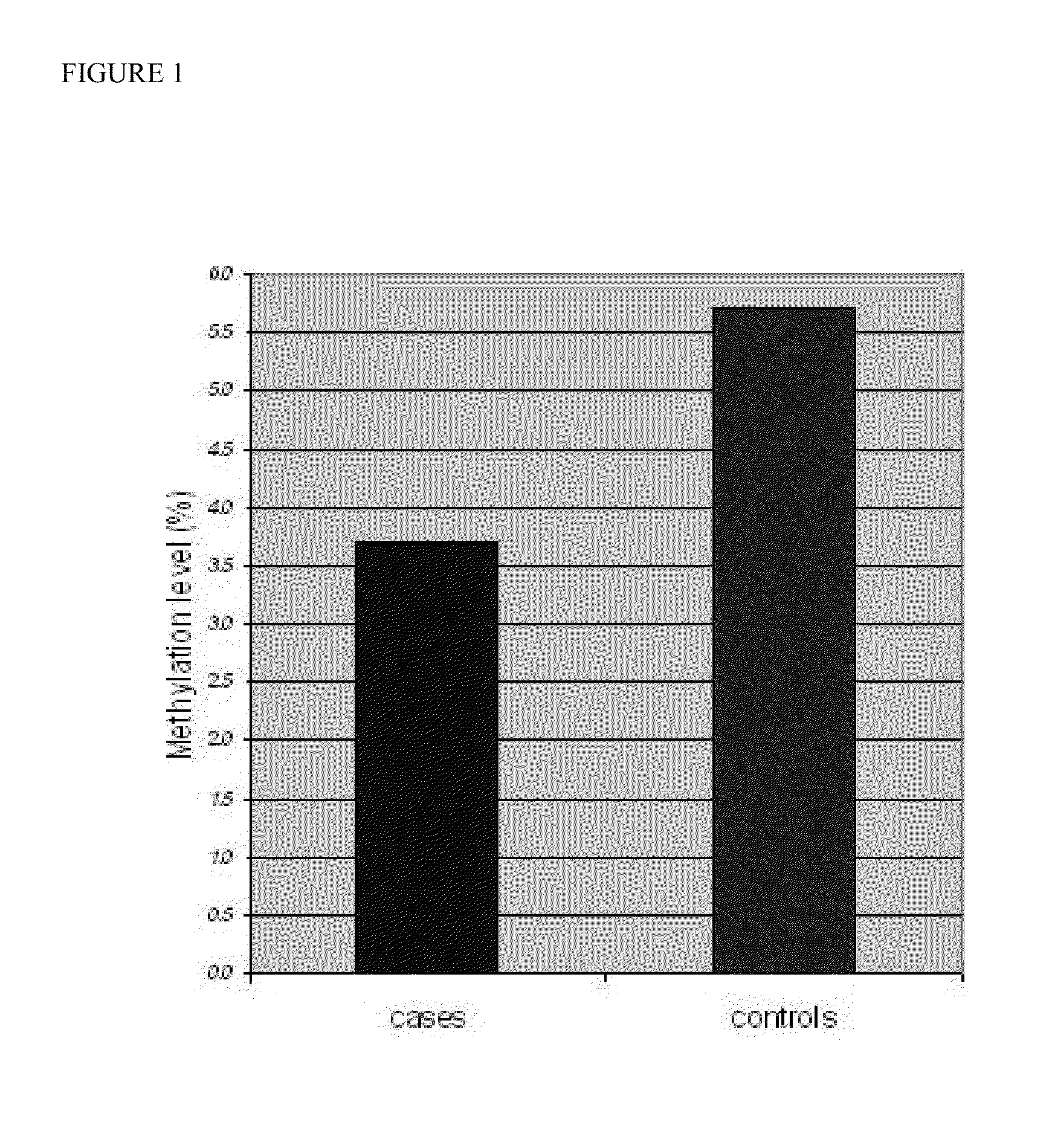
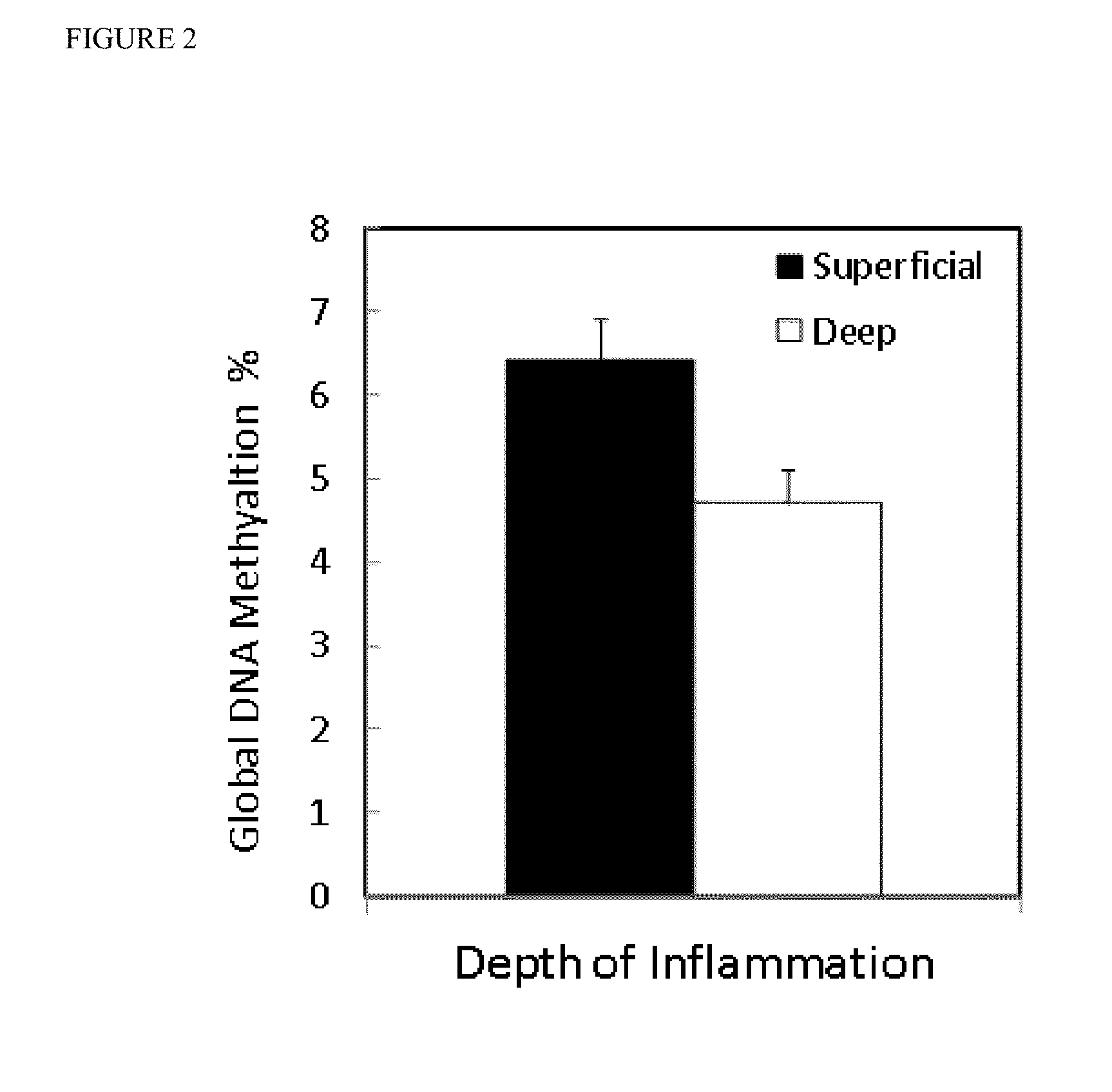
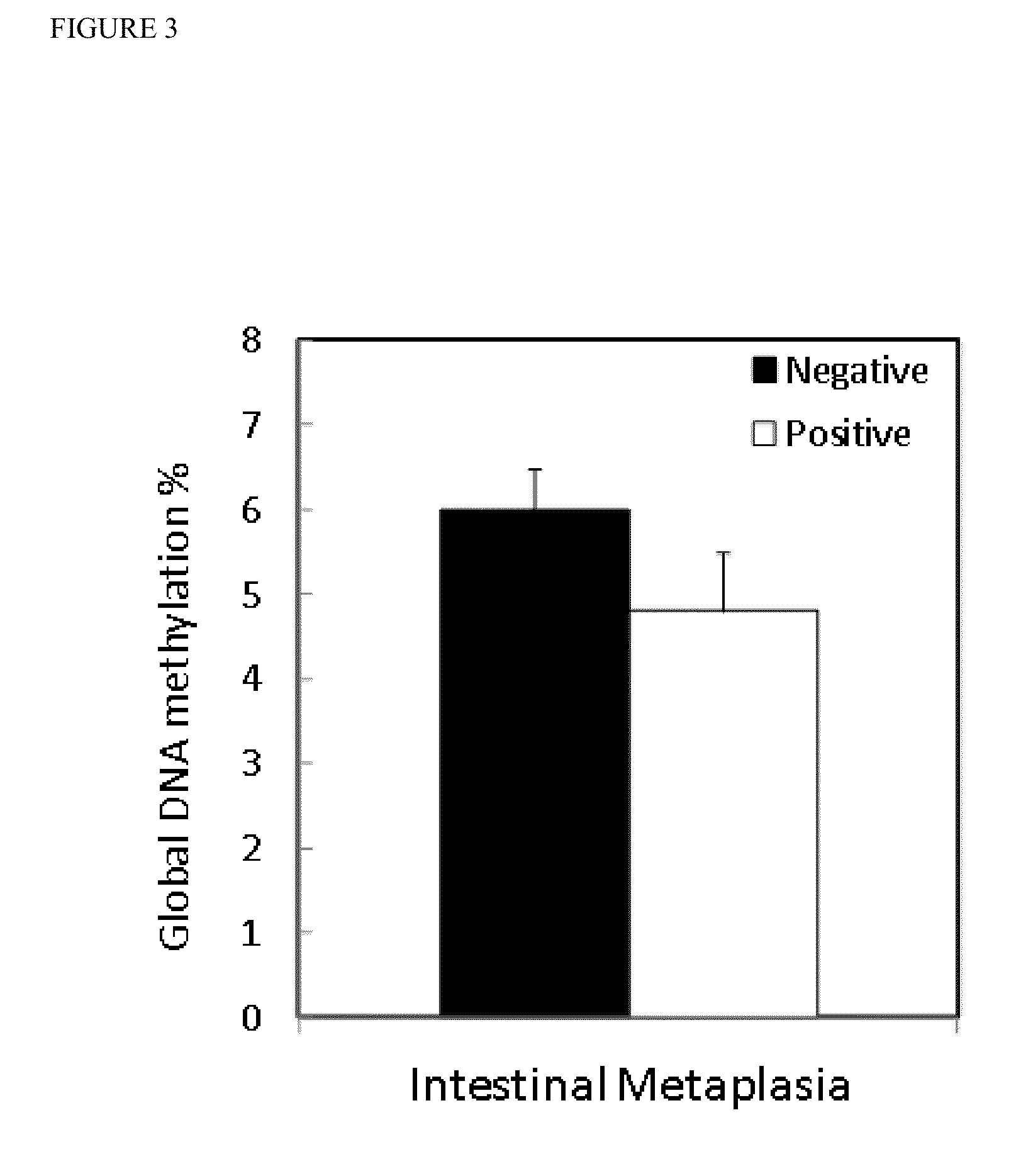
Global DNA hypomethylation and biomarkers for clinical indications in cancer
The present invention provides methods of determination of a global DNA methylation index (GDMI) in a sample from a subject, using a variety of methods which can detect global, genome-wide, and gene-specific DNA methylation to create methylation portraits that can be used for early detection, diagnosis, and clinical management in the personalized medicine space. Further, the invention provides methods of diagnosis of cancer, including gastric cancer and hepatocellular cancer in a subject, by comparing the GDMI in a sample obtained from a subject to the methylation index of standard controls. These methods allow diagnosis of gastric carcinoma and liver cancer in patients who may be asymptomatic or have inconclusive pathology, and allowing earlier treatment of the subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
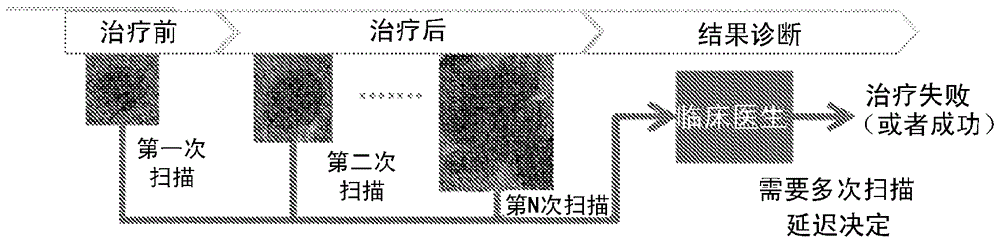
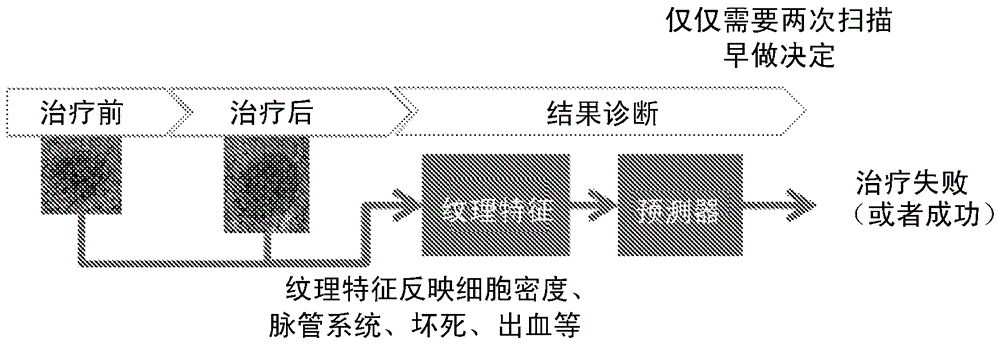
Early therapy response assessment of lesions
For therapy response assessment, texture features are input for machine learning a classifier and for using a machine learnt classifier. Rather than or in addition to using formula-based texture features, data driven texture features are derived from training images. Such data driven texture features are independent analysis features, such as features from independent subspace analysis. The texture features may be used to predict the outcome of therapy based on a few number of or even one scan of the patient
Owner:SIEMENS HEATHCARE GMBH
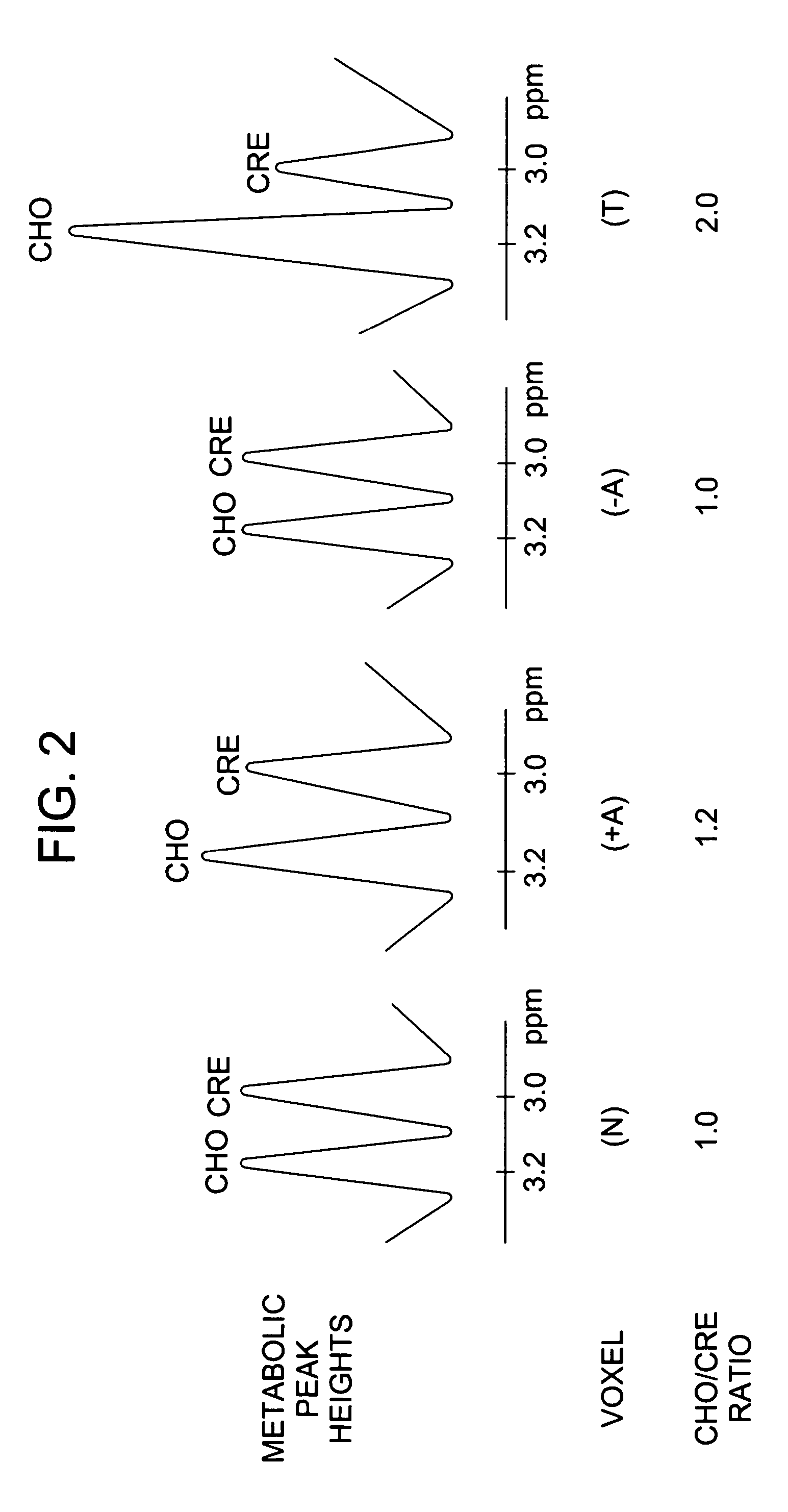
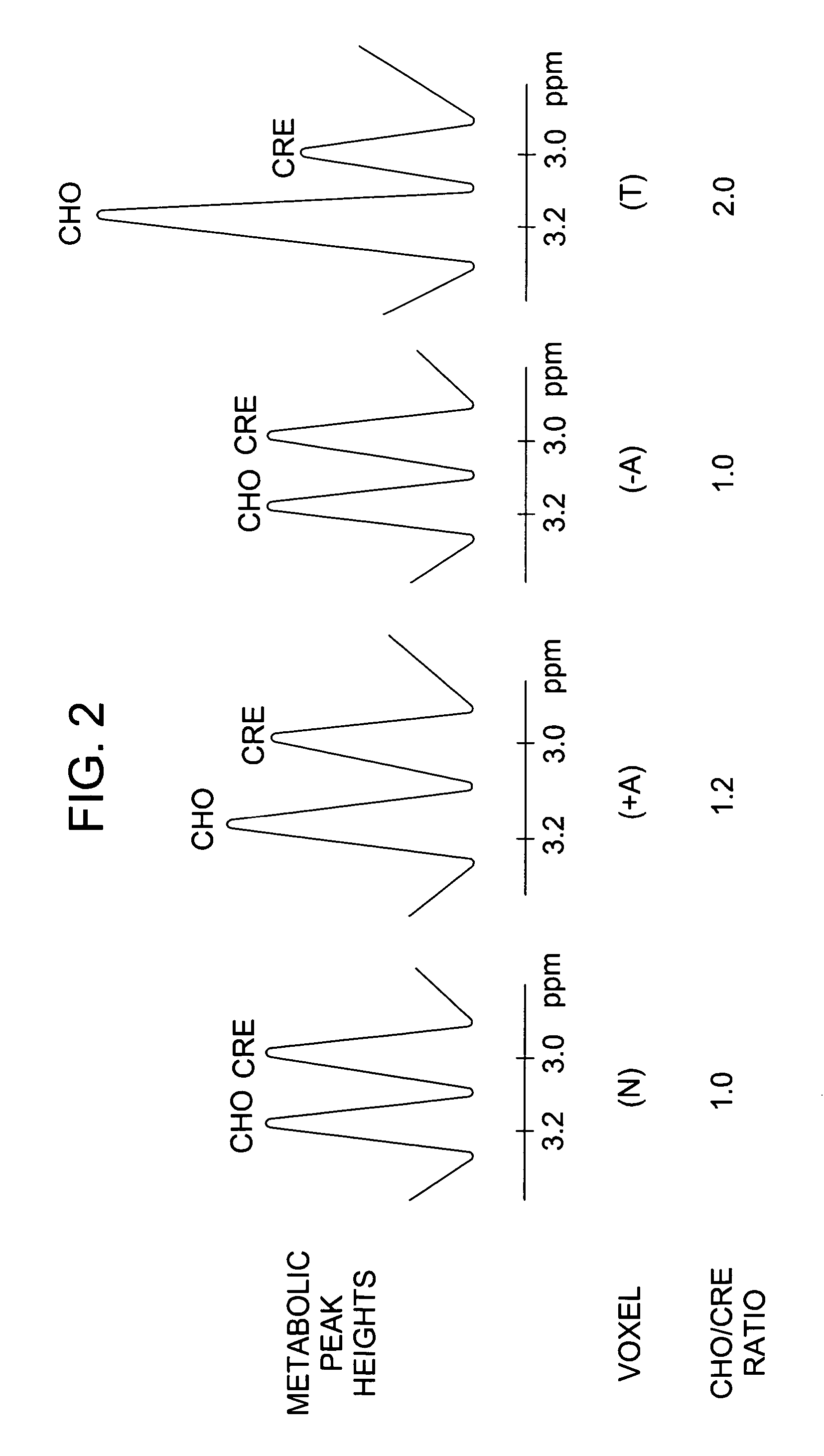
Method for monitoring early treatment response
InactiveUS20060177378A1Early treatment responseMonitor early treatment responseCompounds screening/testingNMR/MRI constrast preparationsCell membranePositive response
Disclosed is a method for monitoring early treatment response of a cancer treatment comprising measuring by magnetic resonance spectroscopy (MRS), for example, proton MRS, the amount of Choline present in the tissue adjoining or surrounding the cancerous tissue before and after treatment; the treatment comprises administration of an angiogenesis inhibitor, for example, a VEGF inhibitor, whereby a decrease in the amount of Choline after treatment is indicative of a positive response. The decrease in the amount of Choline represents the decrease in the internal cell membrane as a result of down regulation of the organelles and their secretory granules and their transport vesicles. Disclosed also is a method for determining effectiveness of an angiogenesis inhibitor in the treatment of cancer. Also disclosed are methods of monitoring early treatment response in diseases where an angiogenesis effector, i.e., an inhibitor or promoter of angiogenesis, is employed. Also disclosed is a method for monitoring protein translation related to angiogenesis.
Owner:RECEPTOMON LLC
Rheumatoid arthritis autoantibody conjugated antigen and applications thereof
The present invention relates to a rheumatoid arthritis autoantibody conjugated antigen and applications thereof. The present invention discloses a novel detection antigen of rheumatoid arthritis antibody, wherein particularly the detection antigen can be used as the RA diagnosis and early diagnosis indicator, and is superior to the clinically developed detections of CCP, CRP, IgG-RF, IgM-RF and the like, such that the detection antigen can be used as the valuable reference indicator for clinical early treatment or prevention of RA, and can further be used as the RA pathogenesis development indicator.
Owner:SHANGHAI EAST HOSPITAL
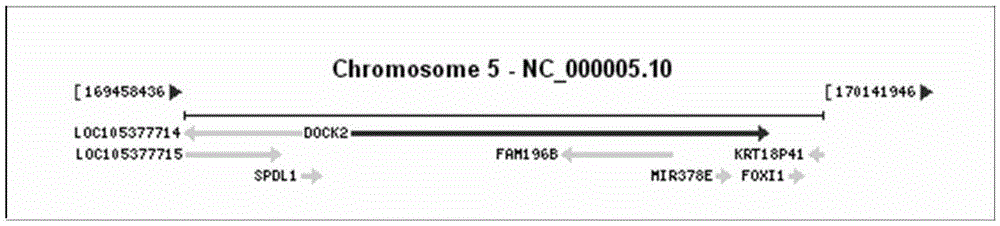
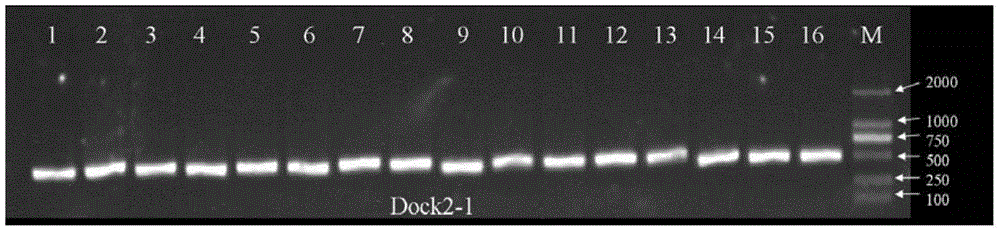
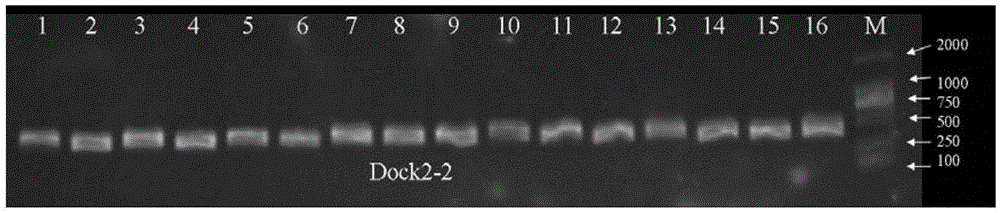
Primers and method for detecting polymorphic hotspot mutation condition of DOCK2 gene
InactiveCN105506107AGood amplification efficiencyThere will be no missed detectionMicrobiological testing/measurementDNA/RNA fragmentationSevere combined immunodeficiency diseaseMutation detection
The invention discloses primers and a method for detecting myelosis myelodysplastic syndrome and especially detecting DOCK2 gene mutation of a patient with myelosis myelodysplastic syndrome. The primers comprise (i) primers for amplifying 6th, 22nd, 33rd, 37th, 40th and 44th exon sequences of the DOCK2 gene. An Sanger sequencing technique and sequencing primers are adopted. The primers and method can be used for quickly detecting mutation of 6th, 22nd, 33rd, 37th, 40th and 44th exons of the DOCK2 gene in the body of a patient with myelosis myelodysplastic syndrome. The primers and method have the advantage of accurate detection result, can be used for assisted diagnosis of severe combined immunodeficiency disease, and have important reference meanings for early intervention and early therapy.
Owner:杭州艾迪康医学检验中心有限公司
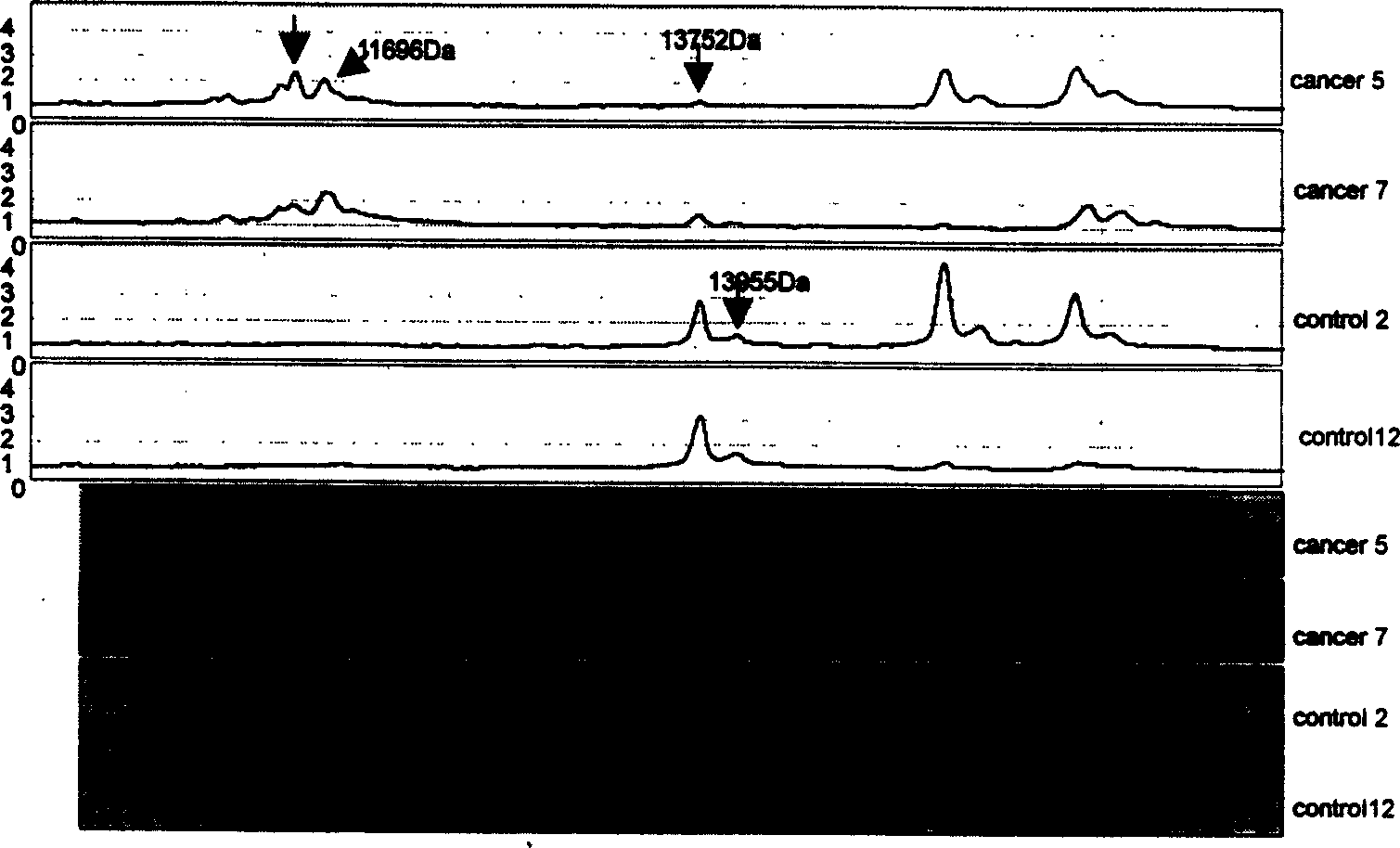
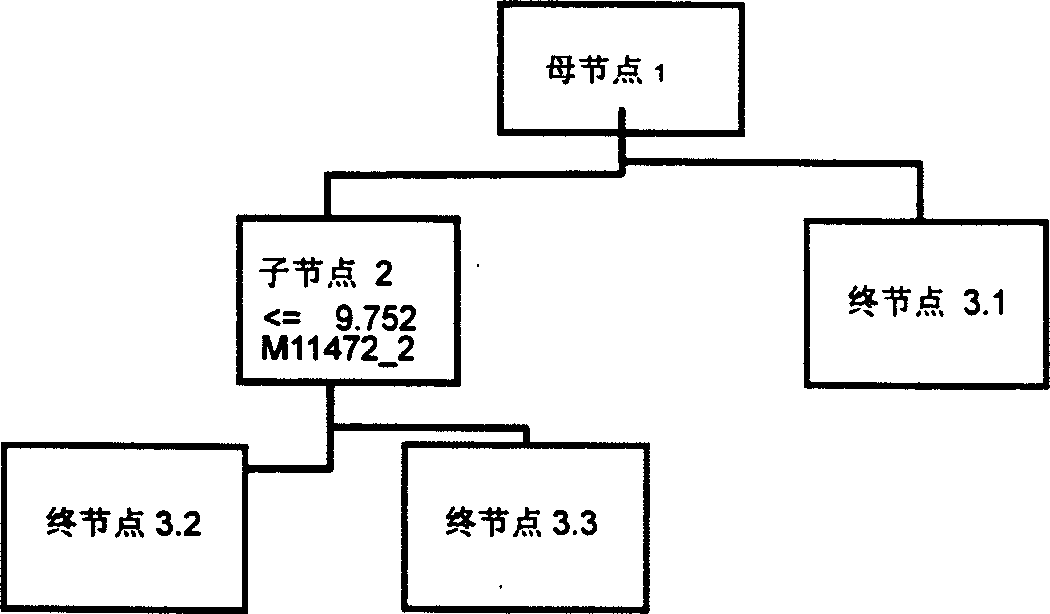
Mass spectrum model for detecting liver cancer serum characteristic protein and method for preparation
InactiveCN1621829AIncreased sensitivityImprove featuresComponent separationBiological testingHigh risk populationsCase fatality rate
The present invention is mass spectral model for detecting liver cancer serum characteristic protein and its preparation process, and belongs to the field of protein fingerprint detecting technology. The present invention screens 17 characteristic proteins including 6 up regulation proteins and 11 down regulation proteins, selects two or more proteins out of the 17 characteristic proteins and establishes two or more stage classification tree with the clinical peak values of the proteins to constitute the detection model for detecting the liver cancer serum characteristic protein. The present invention lays foundation for further discovering new liver cancer biological mark, and the model has over 90 % specificity, sensitivity and positive predicting rate in liver cancer diagnosis.
Owner:THE 306TH HOSPITAL OF PLA
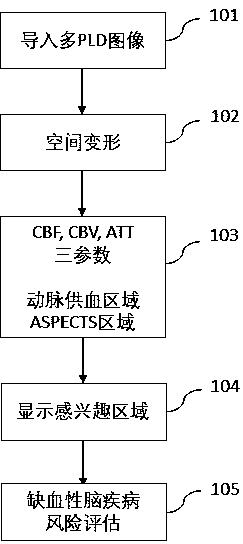
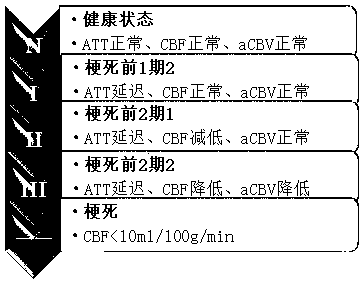
Method for auxiliary assessment of ischemic disease risk based on magnetic resonance cerebral perfusion image
InactiveCN110490871AEffective assessment methodImage enhancementImage analysisHead movementsDisease cause
The invention provides an automatic image processing and displaying method based on a magnetic resonance cerebral perfusion image, which is used for assisting in evaluating cerebral ischemia disease risks and is characterized by comprising spatial deformation, multi-parameterization, brain map coverage and a method for evaluating ischemic cerebral disease risks. The image space deformation methodcomprises the following steps: head movement correction, which is used for adjusting a space difference generated by head movement in a cerebral perfusion imaging process; and standard brain registration: taking the standard brain as a reference to realize standardization of the imported cerebral perfusion image. The multi-parameterization is used for converting the original nuclear magnetic datainto cerebral image perfusion parameters such as cerebral blood flow, cerebral blood volume and artery arrival time, wherein the coverage brain map is used for enabling the parameterized cerebral perfusion image data to cover standard brain maps of different templates to form different regions of interest, and calculating average parameter values of the different regions of interest.The standard cerebral map comprises a cerebral artery blood supply area and an early CT scoring area of an Albetan stroke project. The ischemic brain disease risk assessment method is used for comparing the averageparameter value of the region of interest with a reference range, displaying the differentiation degree and automatically displaying the region of interest beyond the reference range. According to the invention, multi-parameter automatic partitioning and quantitative processing from nuclear magnetic cerebral perfusion original number to ischemic cerebral disease early-stage risk assessment are realized, and an effective assessment method is provided for early-stage screening, early-stage diagnosis and early-stage treatment of cerebral ischemic diseases.
Owner:安影科技(北京)有限公司
Method for monitoring early treatment response
InactiveUS20060177377A1Monitor early treatment responseCompounds screening/testingIn-vivo radioactive preparationsDiseaseCell membrane
Disclosed is a method for monitoring early treatment response of a cancer treatment comprising measuring by magnetic resonance spectroscopy (MRS), for example, proton MRS, the amount of Choline present in the tissue adjoining or surrounding the cancerous tissue before and after treatment; the treatment comprises administration of an angiogenesis inhibitor, for example, a VEGF inhibitor, whereby a decrease in the amount of Choline after treatment is indicative of a positive response. The decrease in the amount of Choline represents the decrease in the internal cell membrane as a result of down regulation of the organelles and their secretory granules and their transport vesicles. Disclosed also is a method for determining effectiveness of an angiogenesis inhibitor in the treatment of cancer. Also disclosed are methods of monitoring early treatment response in diseases where an angiogenesis effector, i.e., an inhibitor or promoter of angiogenesis, is employed.
Owner:RECEPTOMON LLC
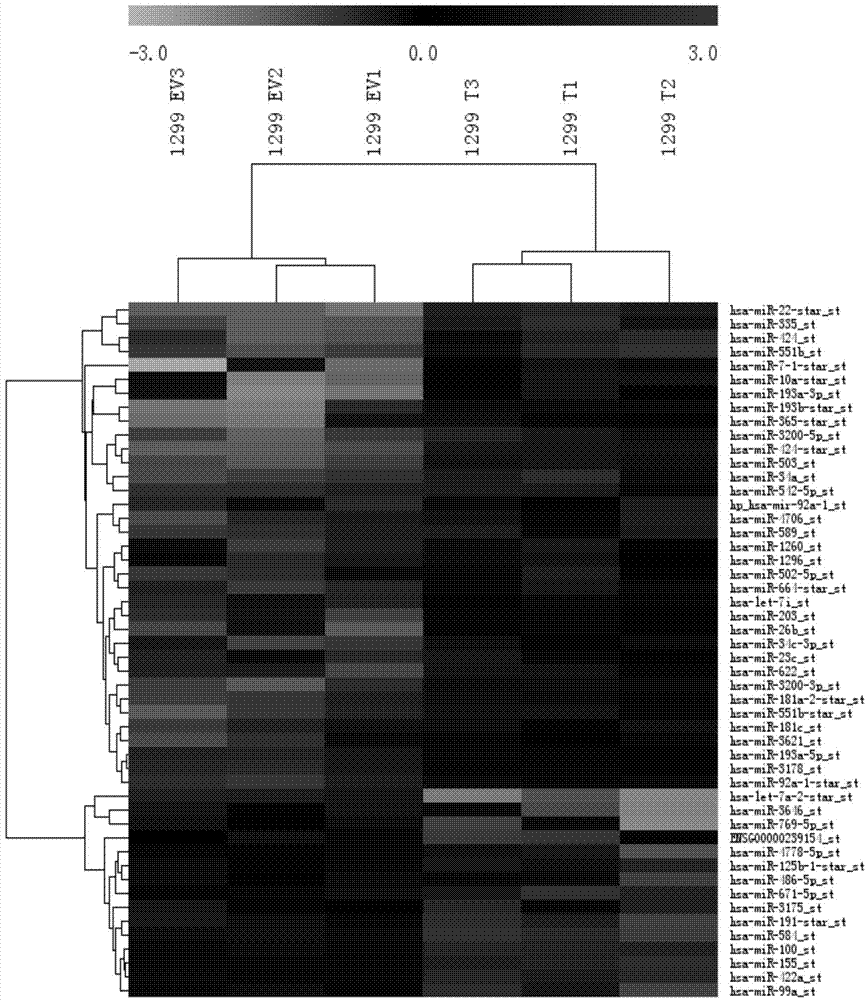
Application of microRNAs in preparation of reagent or kit for early screening or diagnosing Brachyury positive tumors
ActiveCN104498606AProlong lifeReduce mortalityMicrobiological testing/measurementWilms' tumorLung cancer
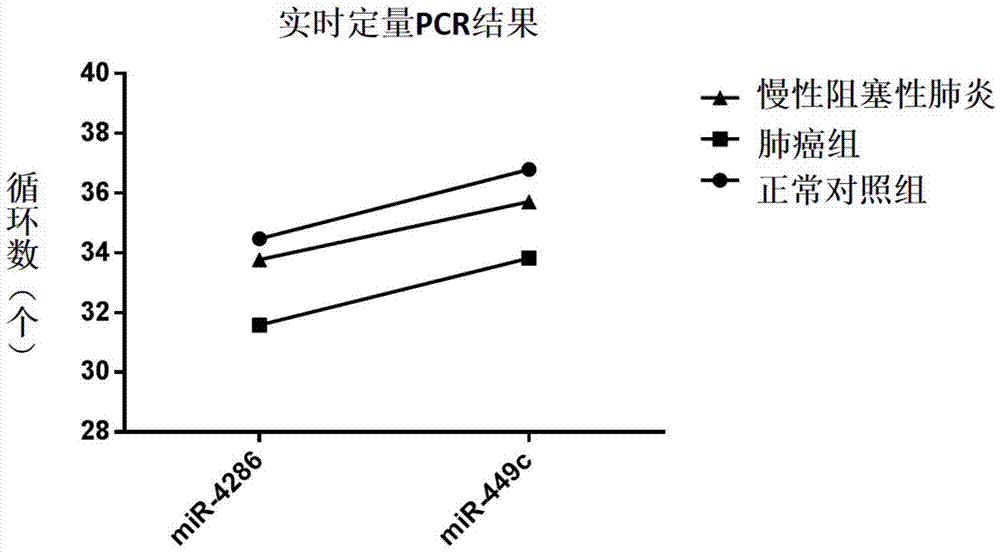
The invention relates to the technical field of medical and biological detection. According to the invention, a plurality of significantly increased microRNAs regulated and controlled by Brachyury are screened by a microRNA chip, and Real-Time PCR validation is carried out on the series of microRNAs. The invention provides a molecular marker of Brachyury positive tumors, and the molecular marker Has-miR-4286, hsa-miR-449c occurs significant high expression in lung cancer patients and is free of obvious abnormal expression level in other lung solid lesion patients or normal people. The invention further provides an application of microRNAs in preparation of a reagent or a kit for early screening or diagnosing Brachyury positive tumors. The microRNAs provided by the invention can be used for screening or diagnosing tumors at the early stage and has important significance on the early treatment of tumors and saving the medical cost.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
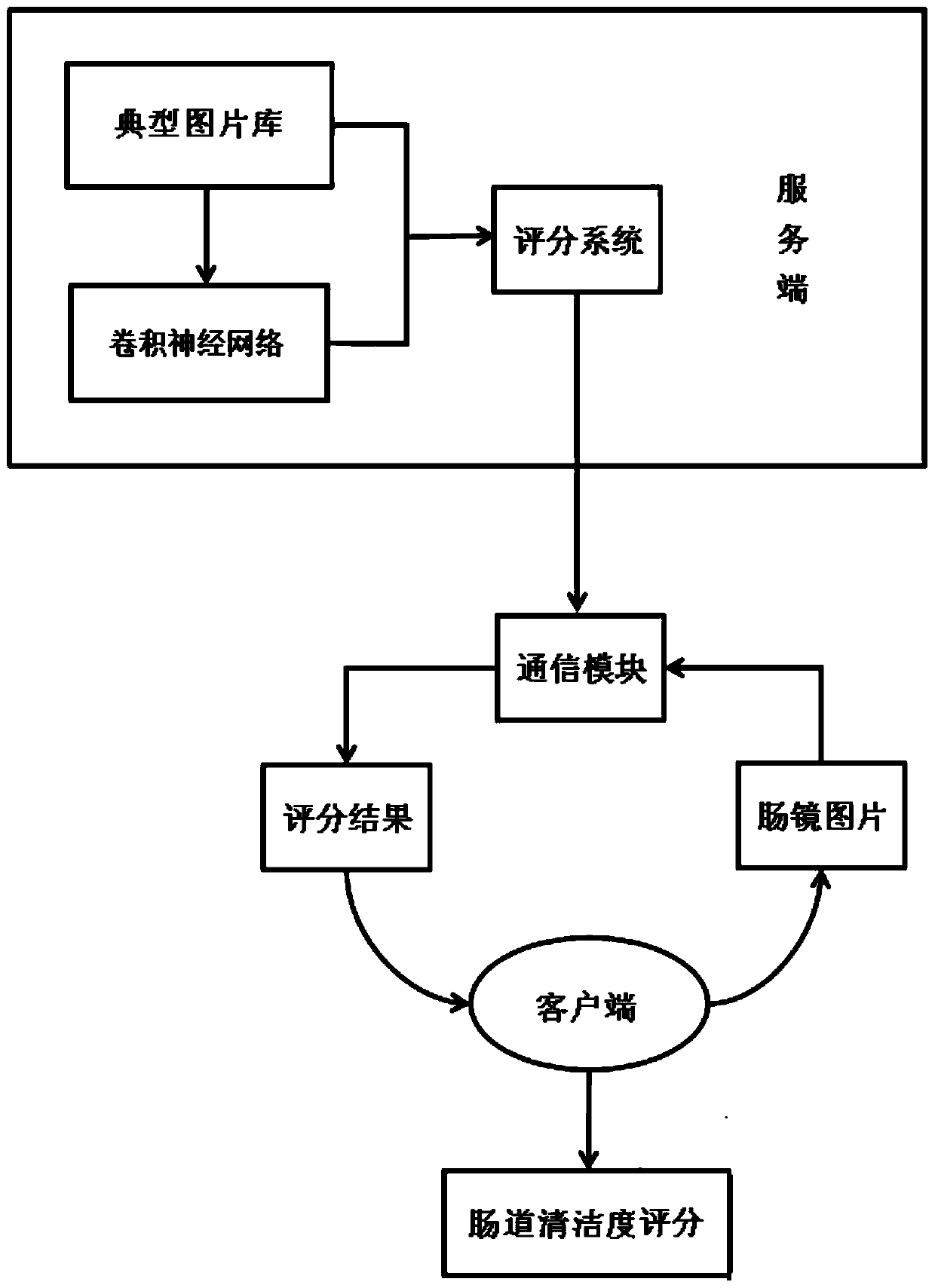
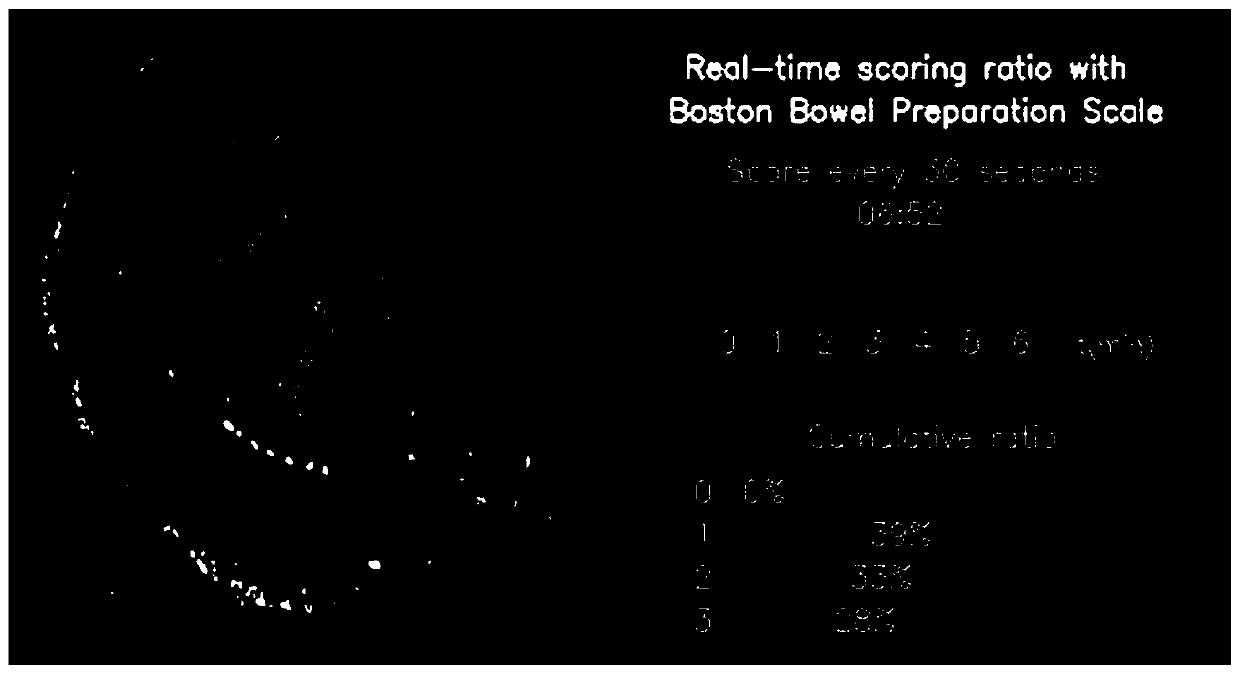
Real-time intestinal tract cleanliness scoring system and method based on artificial intelligence
InactiveCN110916606APrepare for quality understandingImprove preparation qualityEndoscopesRectum colonoscopesBowel preparationPrecancerous condition
The invention discloses a real-time intestinal tract cleanliness scoring system and method based on artificial intelligence. By adopting the system and method, bowel preparation quality examined by aclinical colonoscope is monitored in real time, and scoring display is carried out at a client; and the constituent ratio of each score and the cleanliness of an examined intestinal segment within each 30 s are represented. On one hand, the bowel preparation quality condition of a patient operated by a physician can be more objectively and directly expressed in a quantitative manner, and the workload and scoring errors of endoscopic physicians are reduced, so that the error evaluation of endoscopic examination quality is reduced and the interval of reexamination of colonoscope examination isrecommended; on the other hand, medical institutions can more objectively and directly know about the bowel preparation quality, so that the quality control work can be done effectively, the intestinal tract cleaning quality is rapidly improved, the adenoma detection rate is reduced, and early detection and early treatment of precancerous lesions of intestinal tracts are realized; and more importantly, the development of the system facilitates scientific inquiry for subsequent influences on bowel preparation schemes by different intestinal tract preparations.
Owner:WUHAN ENDOANGEL MEDICAL TECH CO LTD
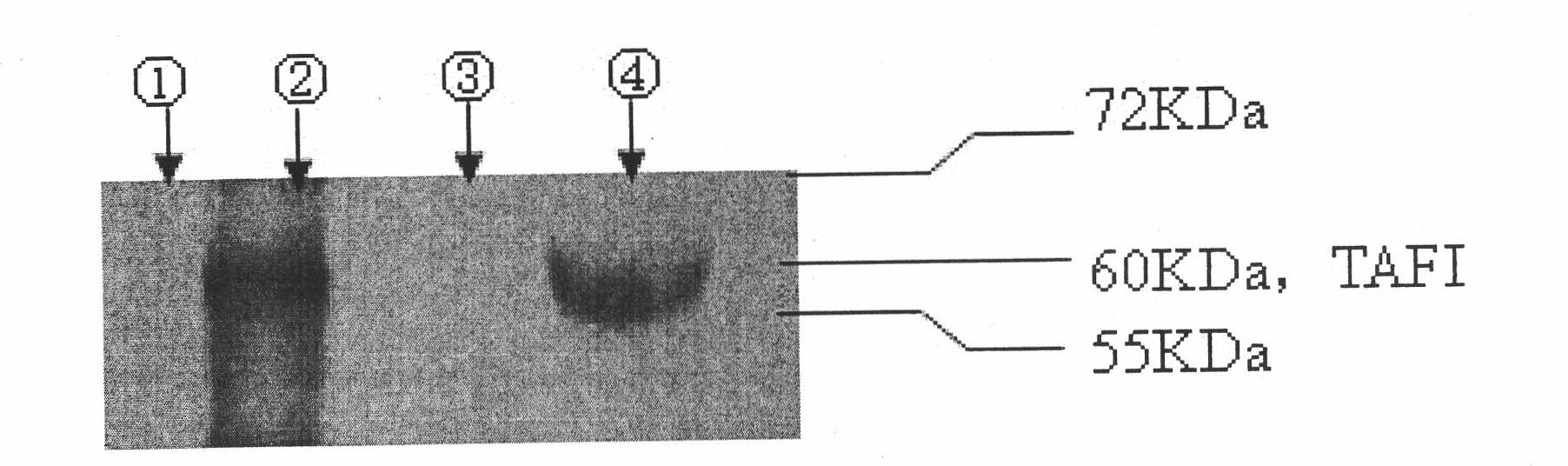
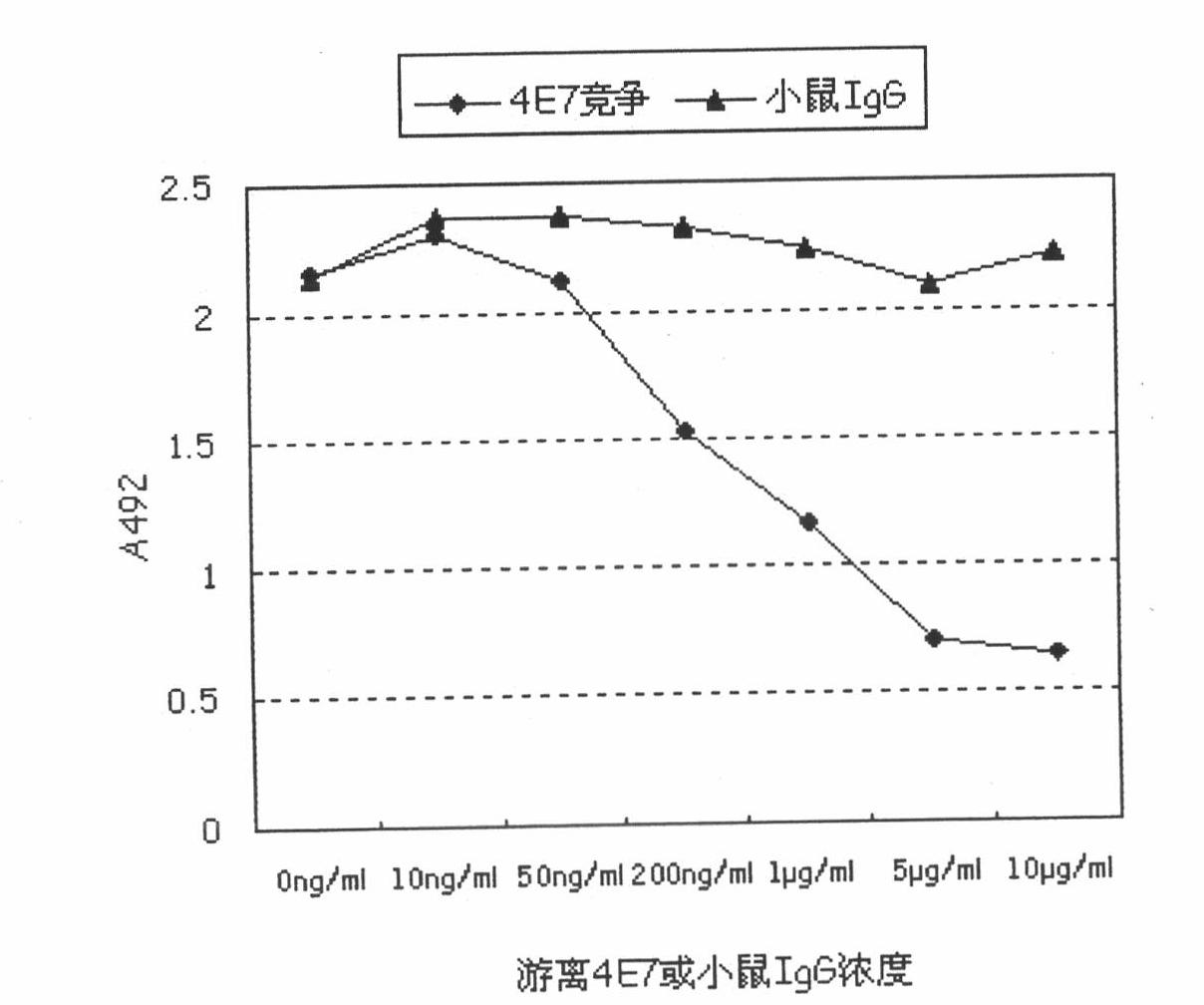
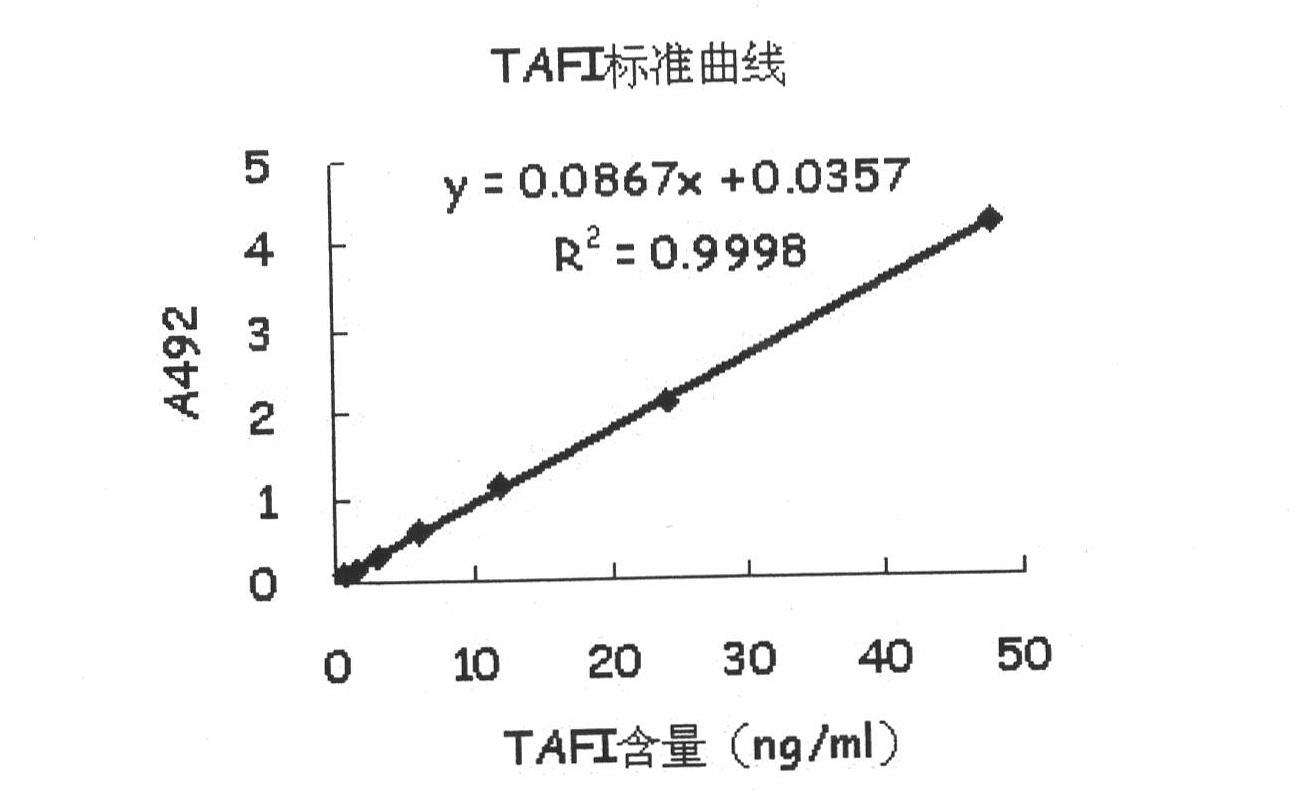
In-vitro assay method for thrombin-activatable fibrinolysis inhibitor (TAFI) content
ActiveCN102103142ASolve defects such as expensive and long detection timeSimple and fast operationPreparing sample for investigationDendrimerDisease
The invention discloses an in-vitro assay method for thrombin-activatable fibrinolysis inhibitor (TAFI) content. The method is used for in-vitro assay of the TAFI content by using the biological characteristic that polyamidoamine (PAMAM) dendrimer can be combined with an antibody in combination with the conventional enzyme linked immunosorbent assay (ELISA) method to develop a new in-vitro diagnostic method for a TAFI, so that the flexibility of TAFI in-vitro assay is improved. In the method, a TAFI content in-vitro assay kit comprises an antibody-coated elisa plate, sample diluent, a TAFI standard product, cleaning solution, a PAMAM-labeled horseradish peroxidase bound antibody, antibody diluent, color development buffer solution, 30 percent hydrogen peroxide, o-phenylendiamine and stop solution. The defects of expensive equipment, longer assay time and the like existing in the prior art are overcome; compared with methods using other assay kits, the method has the advantages of low cost, sensitivity of diagnosis, low detection limit and the like, is easy and convenient to operate quickly and is used for auxiliary diagnosis, the detection rate and the accuracy of diseases are improved, and the aims of early detection and early treatment are fulfilled; and unnecessary pain and medical expenditure of an assayed person are reduced, and the quality of life of the assayed person is improved.
Owner:LIAONING MEDI BIOTECH CO LTD
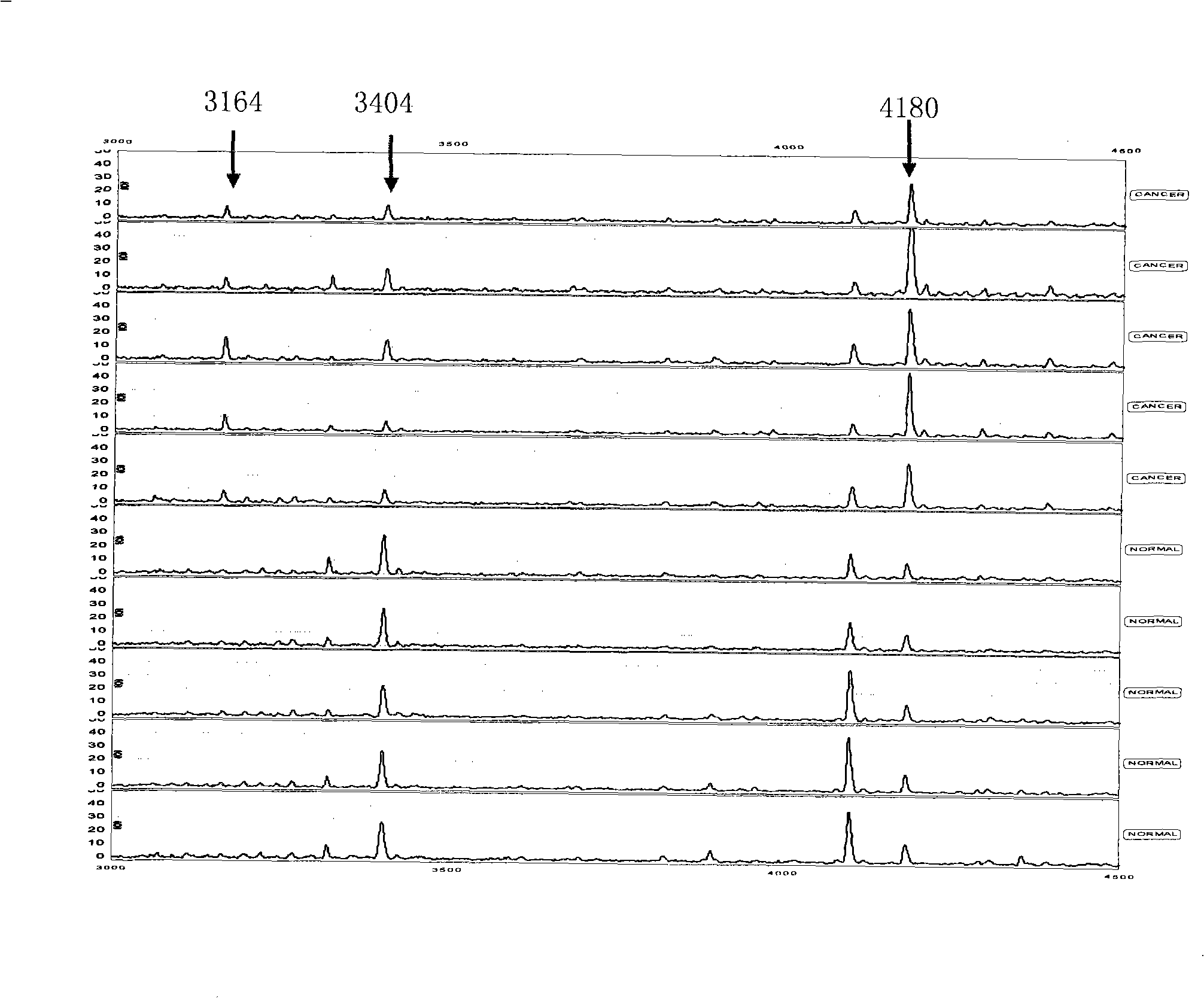
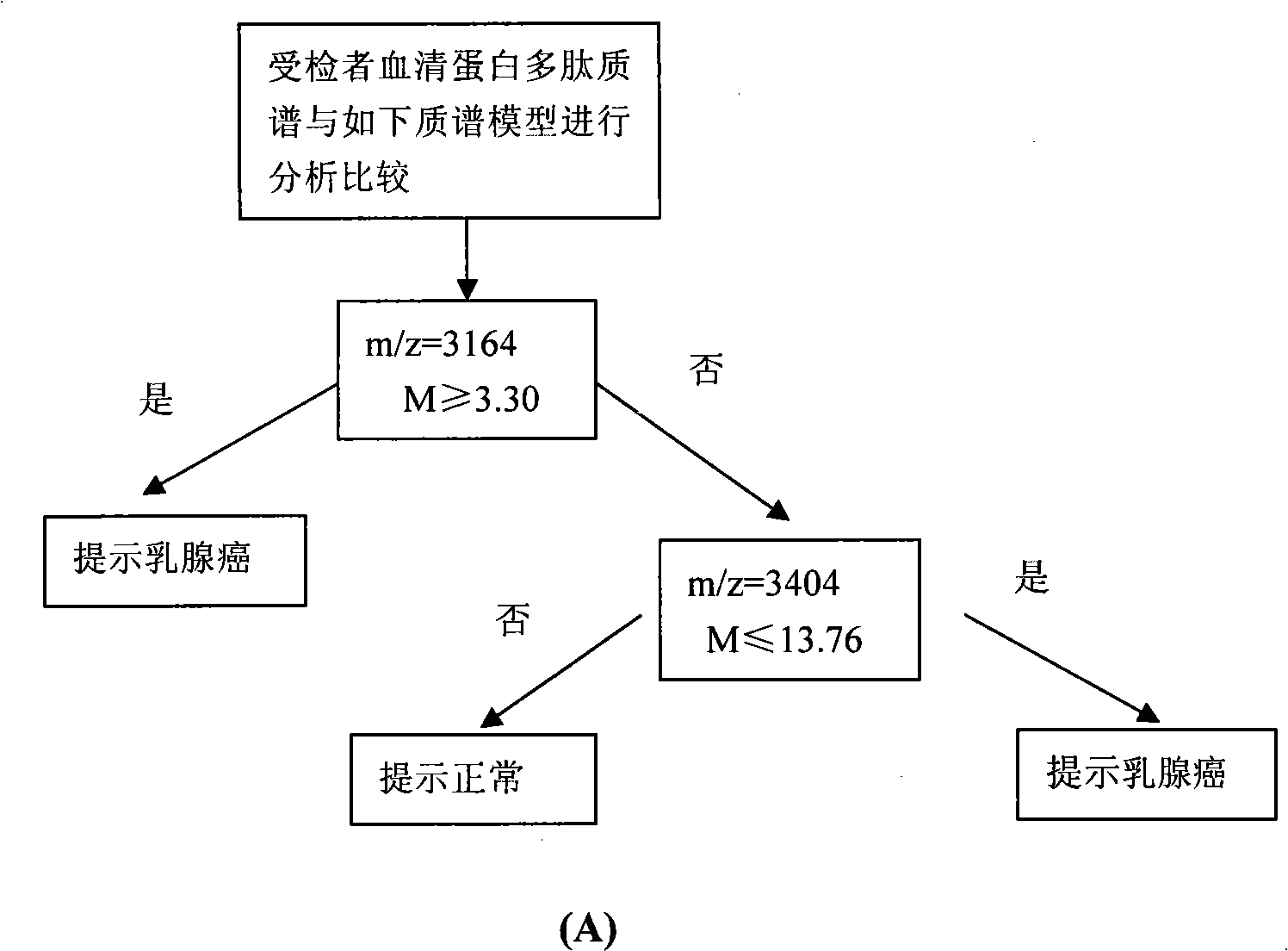
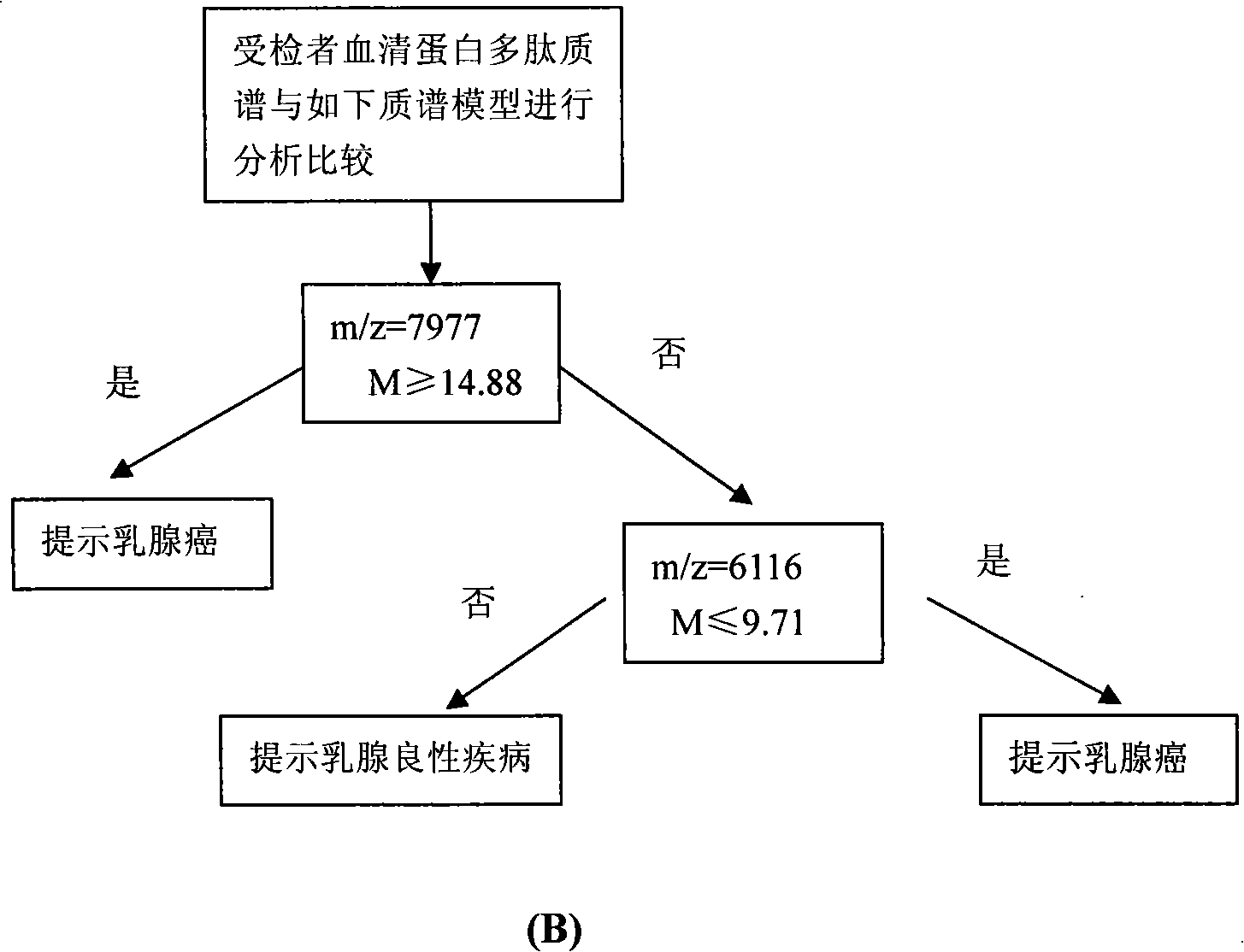
Optimizing mass spectrogram model for detecting breast cancer characteristic protein and preparation method and application thereof
InactiveCN101329346AIncreased sensitivityImprove featuresComponent separationBiological testingDiseaseHigh risk populations
The invention relates to an optimum mass spectrometry model and a preparation method thereof for detecting the feature protein of breast cancer, belonging to the field of mass spectrometry detection technique. The invention is characterized in that eight up-regulated proteins and three lower-regulated proteins are screened from the blood serum to be used as the feature proteins; any two or more proteins of the eleven proteins are chosen so as to establish a blood serum feature protein mass spectrometry model of identification with two in a group for patients with breast cancer and normal people, and patients with benign breast disease, lymphatic metastasis of breast cancer and remote metastasis of breast cancer according to the mass-charge ratio m / z of each protein peak and the critical peak average value of the protein; the preparation method of the invention provides a foundation for further discovering new breast cancer biological marks. The method of the invention is better than any single detection method adopted currently for the detection of the breast cancer, and provides a non-invasive technique for the early detection and early treatment of the breast cancer, thus providing a new method for reducing the mortality of the breast cancer, improving the cure rate of the breast cancer and screening and examining the breast cancer for high-risk population further.
Owner:许洋
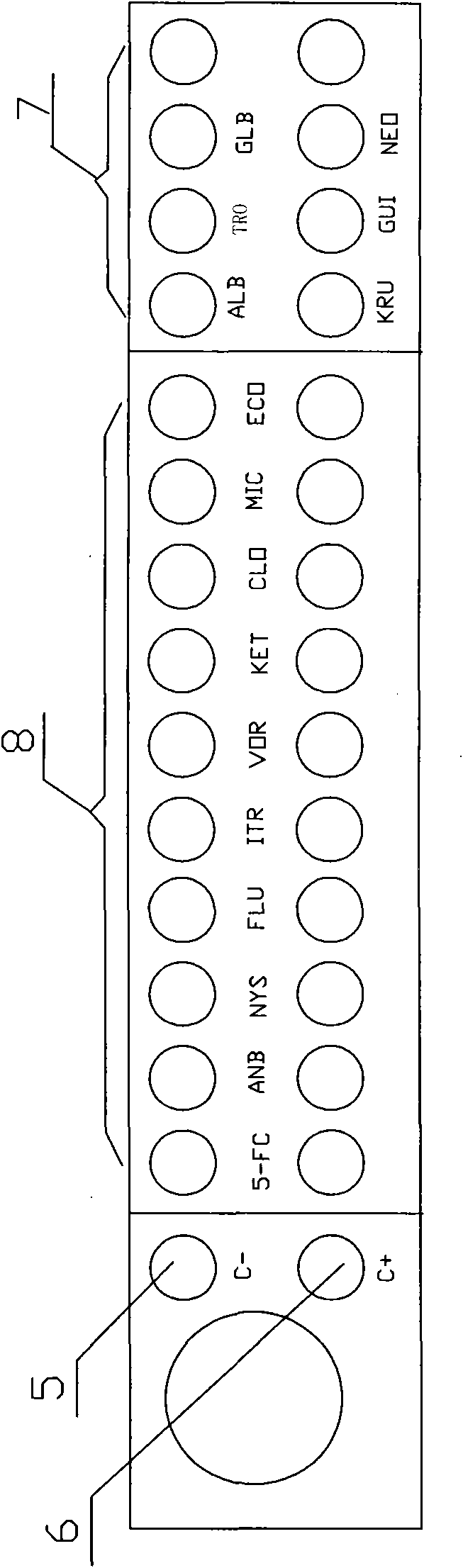
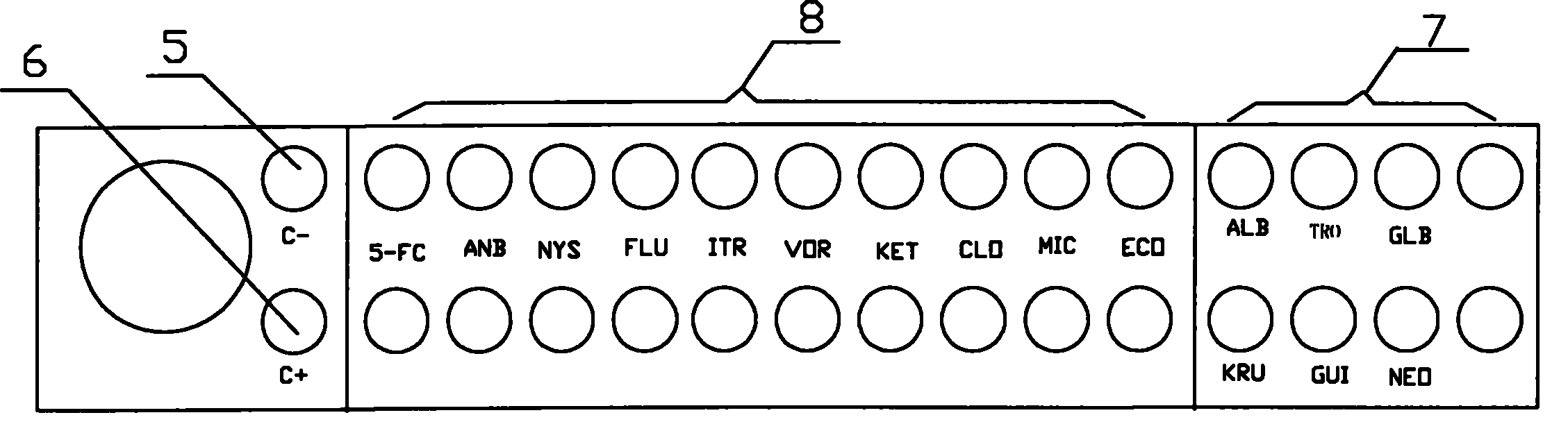
Fungus rapid culture, identification and susceptibility detection kit and detection method thereof
ActiveCN101886111AGrowth inhibitionGrow fastMicrobiological testing/measurementCandida famataAntibiotic Y
The invention discloses a fungus rapid culture, identification and susceptibility detection kit for rapidly and effectively identifying multiple fungi and testing the susceptibility. The invention also discloses a method for directly detecting a clinical sample by using the kit. The kit has the advantages that the multiple fungi are simultaneously identified and different pores are favorable for growing different fungi by coating various sugars or inhibitors on a susceptibility test board, so that the most common fungi in clinic use, such as candida albicans, candida tropicalis, candida glabrata, candida krusei, cryptococcus neoformans, candida guilliermondii and the like, can be identified and effective guidance is provided for the clinical use. The detection method is simple and suitable for detecting pure fungi, can be further directly applied to fungus identification of the clinical sample and the susceptibility detection of antibiotics and has a short detection period, namely thefungi to be detected can be simultaneously identified and the susceptibility can be detected at one time and the result can be given in 24 hours; and thus the detection method greatly shortens the clinical detection period and is favorable for early diagnosis and early therapy of patients.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Yeast cDNA library of hog-cholera-virus-resisting VHH antibody as well as construction method and applications of yeast cDNA library
InactiveCN107304419AGood varietyImmunoglobulins against virusesMicroorganism librariesTiterClassical swine fever
The invention discloses a yeast cDNA library of a hog-cholera-virus-resisting VHH antibody as well as a construction method and applications of the yeast cDNA library. According to the yeast cDNA library, the construction method and the applications, the peripheral blood of a camel which is immunized by a hog cholera C strain attenuated vaccine is separated, then lymphocytes are obtained, the total mRNA of the lymphocytes obtained through separation is extracted, cDNA fragments of the hog-cholera-virus-resisting VHH antibody are obtained through an RT-PCR method, the cDNA fragments of the VHH antibody and pGADT7-Rec co-transform yeast competent cells, and thus the yeast cDNA library of the hog-cholera-virus-resisting VHH antibody is obtained. The capacity and polymorphism of the constructed yeast cDNA library are identified, the result shows that the titer of the library is about 5.5x10<6>cfu / ml, the diversity is good, and the recombination rate of the library is 100%. Preliminary functional identification is carried out on the yeast cDNA library of the hog-cholera-virus-resisting VHH antibody by adopting a blood coagulation inhibition test, and the result shows that the hog-cholera-virus-resisting VHH antibody with the neutralizing activity exists in the constructed yeast library. The proposal of the technical scheme provides a platform for screening the hog-cholera-virus-resisting VHH antibody, and a novel technical means is provided for early treatment and diagnosis of hog cholera.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
System and method for diagnosis and early treatment adoption for asymptomatic disease
Systems and methods are provided for diagnosis of and early treatment adoption for asymptomatic diseases. In one embodiment, an examination system is provided including an input device for entering patient data, a computer including a medium for storing entered data, and a feedback module for providing feedback to an examined patient based on the data entered. A method of using the examination system is also provided in which feedback is provided directly to the patient concurrently with the measurement or other acquisition of a significant data point. Feedback is also provided at the conclusion of the examination in the form of a report, treatment plan, or customized educational materials. In a further embodiment, the patient is an active participant in the examination, recording data points themselves via the input device.
Owner:GIBBS CHRIS
Method for monitoring early treatment response
InactiveUS20060064003A1Monitor early treatment responseMechanical/radiation/invasive therapiesMagnetic measurementsCell membranePositive response
Disclosed is a method for monitoring early treatment response of a cancer treatment comprising measuring by magnetic resonance spectroscopy (MRS), for example, proton MRS, the amount of Choline present in the cancerous tissue before and after treatment; the treatment comprises administration of a cell surface receptor inhibitor, for example, an EGFR inhibitor, whereby a decrease in the amount of Choline after treatment is indicative of a positive response. The decrease in the amount of Choline represents the decrease in the internal cell membrane as a result of down regulation of the organelles and their secretory granules and their transport vesicles. Disclosed also is a method for determining effectiveness of a cell surface receptor inhibitor in the treatment of cancer.
Owner:RECEPTOMON LLC
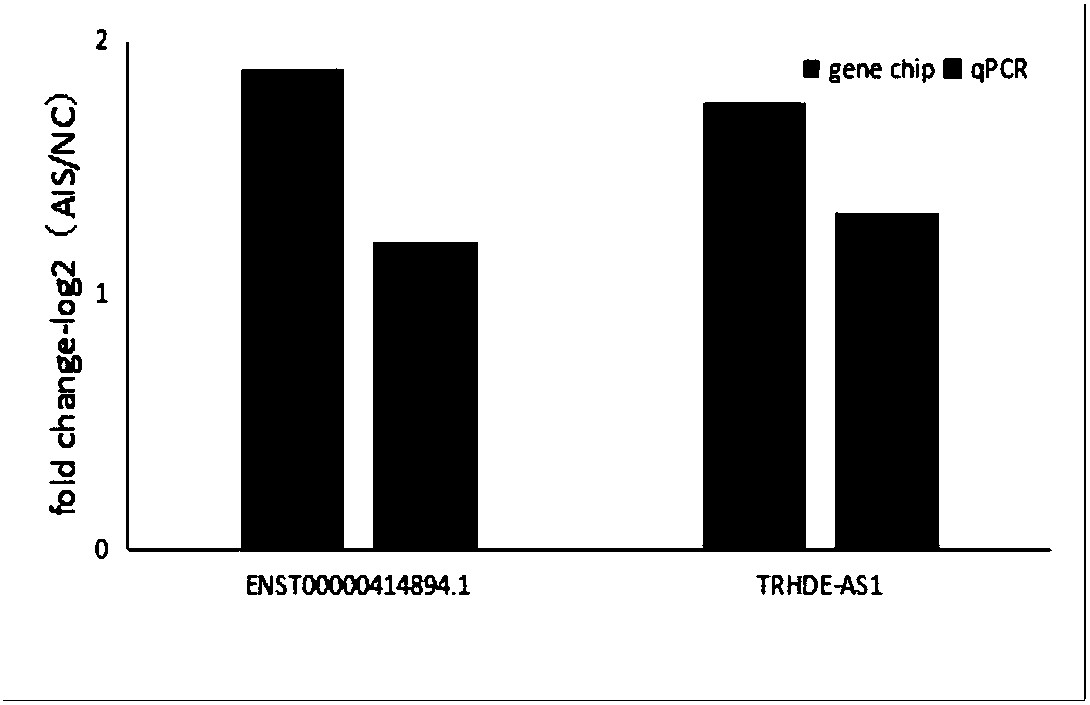
Applications of IncRNA in preparation of products which are used for diagnosis or prediction of adolescent idiopathic scoliosis
ActiveCN108130368ASave treatment cosSave medical costMicrobiological testing/measurementDNA/RNA fragmentationMedicineTreatment targets
The invention discloses novel molecular markers ENST00000414894.1 and / or TRHDE-AS1 which are related to adolescent idiopathic scoliosis. It is confirmed that expression down-regulate of ENST00000414894.1 and TRHDE-AS1IncRNA in patients with adolescent idiopathic scoliosis is induced, so that applications in preparation of products which are used for diagnosis or prediction of adolescent idiopathicscoliosis are realized. The invention also discloses applications of the molecular markers in preparation of kits which are used for diagnosis or prediction of adolescent idiopathic scoliosis. The molecular markers can be used for rapid and effective diagnosis of early stage adolescent idiopathic scoliosis. Significant importance on early stage treatment and chemical cost reduction of adolescentidiopathic scoliosis is achieved, and treatment targets and important basis are provided for clinical applications such as gene treatment and medicine treatment.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Kit for screening dilated cardiomyopathy
ActiveCN103509867AImprove survival rateImprove early detection rateMicrobiological testing/measurementMYPNBiology
The invention relates to a kit for screening dilated cardiomyopathy. The following four detection genes are used in the kit: MYBPC3, SCN5A, MYH7 and MYPN. The invention also provides the application of the four genes (MYBPC3, SCN5A, MYH7 and MYPN) in preparing the kit for screening dilated cardiomyopathy. The invention has the following advantages: the effective method and the kit for early diagnosis of sporadic dilated cardiomyopathy in the Han nationality in China are developed by screening the four genes causing sporadic dilated cardiomyopathy which are most susceptible in the Han nationality in China, so that the early screening rate of patients with dilated cardiomyopathy is effectively increased to support early treatment and increase the survival rate of patients; compared with the expensive and low-efficiency dilated cardiomyopathy gene screening method in the prior art, the discovery of the four high-risk pathogenic genes enables the screening to be more targeted, so as to reduce the examination expenses and increase the detection efficiency.
Owner:苏州科诺医学检验实验室有限公司
Inhibition of rho and or rock and cell transplantation
InactiveUS20150297643A1Improving cell transplantationImprove survivabilityBiocidePeptide/protein ingredientsInjury SiteCombined treatment
The present disclosure provides a multi-treatment combination to improve recovery after spinal cord injury or neurotrauma comprising: (a) Cell transplantation at the site of spinal cord injury and (b) Surgical delivery of BA-210. The combined treatment allows cells to be transplanted in the injury site during the acute trauma period, a time when the inflammatory response to neurotrauma adversely effects survival of transplanted cells. Early therapy delivered during critical care treatment after neurotrauma is essential for successful restorative therapy. The multi-treatment combination also provides a method to ensure that multi-potent transplanted cells do not become tumorigenic.
Owner:BIOAXONE BIOSCI
Kit for screening dilated cardiomyopathy genetic susceptibility genes
InactiveCN106191290AImprove early detection rateImprove survival rateMicrobiological testing/measurementDiseaseExon
The invention discloses a kit for screening dilated cardiomyopathy genetic susceptibility genes. The kit is used for detecting three genes of MYBPC3, SCN5A and MYH7 and reagents for conducting complete exon sequencing on MYBPC3, SCN5A and MYH7. According to the kit for screening the dilated cardiomyopathy genetic susceptibility genes, the most susceptible sporadic dilated cardiomyopathy disease-causing genes of people are screened out, the kit which is effective to dilated cardiomyopathy is developed out, the early diagnosis rate of the dilated cardiomyopathy is effectively increased, and therefore early treatment is achieved, and the survival rate of patients is increased.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
Molecular marker relevant to adolescent idiopathic scoliosis and application thereof
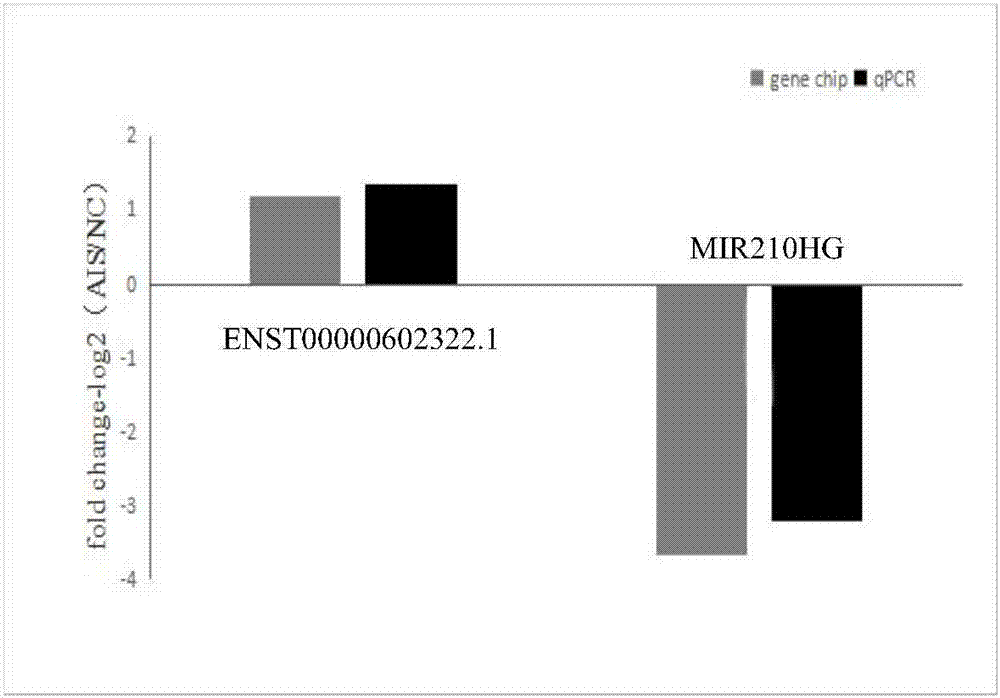
The invention discloses a molecular marker ENST00000602322.1 and / or MIR210HG relevant to adolescent idiopathic scoliosis, and further verifies that ENST00000602322.1 represents down-regulated repression in adolescent idiopathic scoliosis, and MIR210HG represents up-regulated expression in adolescent idiopathic scoliosis, and therefore, the molecular marker can be applied to preparation of productsfor diagnosing or predicating adolescent idiopathic scoliosis. Meanwhile, the invention further discloses an application of the molecular marker in preparation of a kit for diagnosing or predicatingadolescent idiopathic scoliosis. By using the molecular marker, early diagnosis of adolescent idiopathic scoliosis can be realized, thus being rapid and effective, not only having significant meaningfor early treatment of adolescent idiopathic scoliosis and saving medical cost, but also providing therapeutic targets and important basis for clinical application for gene therapy and drug therapy.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Novel use of antihistamine agents for the preventive or early treatment of inflammatory syndromes, in particular those triggered by togaviruses
The present invention relates to the use of at least one antihistamine agent for the preparation of a medicament for use in the preventive or early treatment of inflammatory syndromes of viral origin, in particular arthritis of the distal joints, more particularly those triggered by togaviruses. The invention also relates to a combination product of at least one antihistamine agent and of at least one antiserotonin agent for its simultaneous, separate or sequential use in preventive or early therapy for inflammatory syndromes of viral origin, in particular arthritis of the distal joints, more particularly those triggered by togaviruses.
Owner:PIERRE FABRE MEDICAMENT SAS
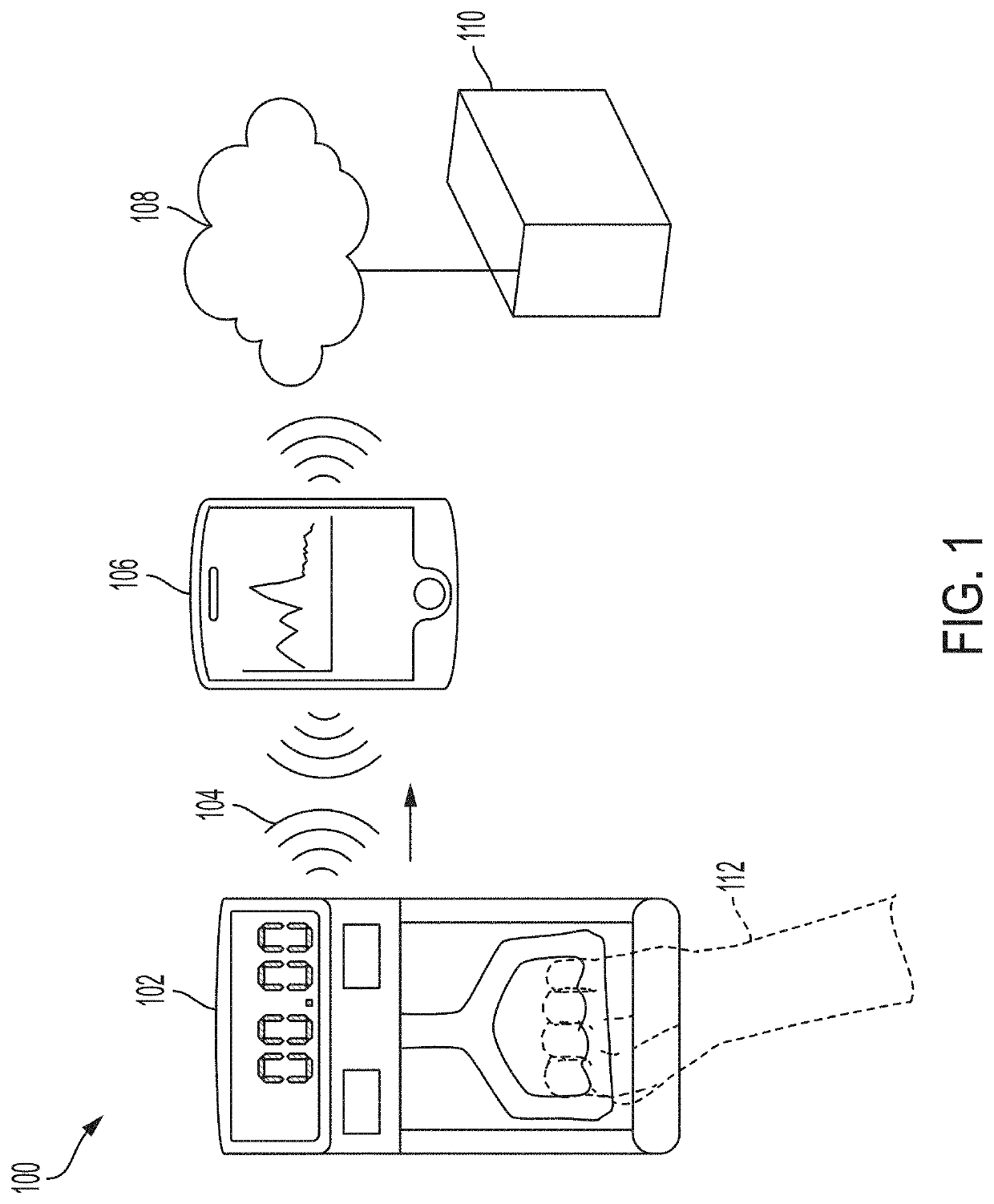
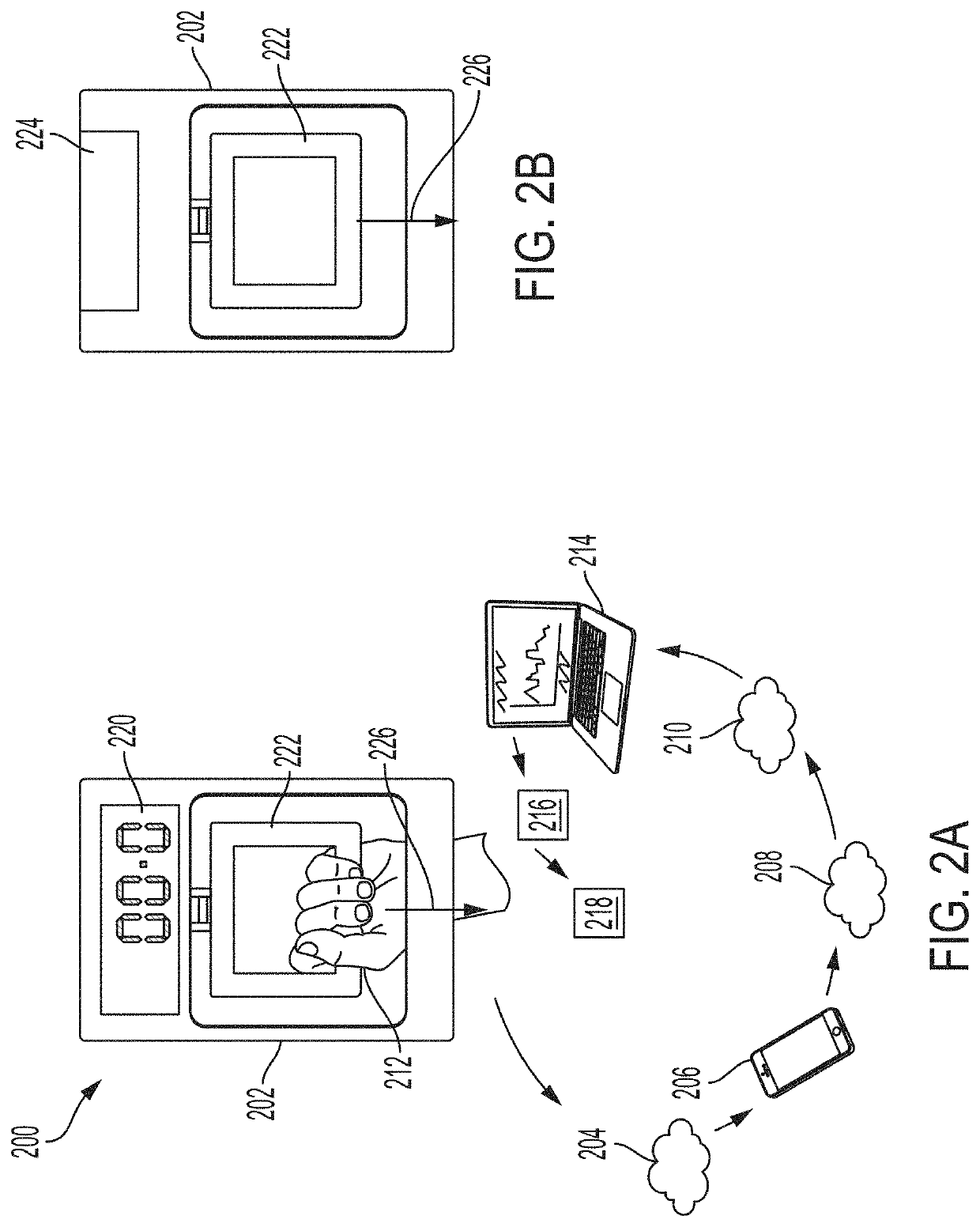
System and methods for remotely monitoring lean muscle mass
ActiveUS20200129107A1Easy to trackImprove analysisPhysical therapies and activitiesSensorsDiseasePhysical medicine and rehabilitation
Improved systems and methods for improving lean muscle mass monitoring of patients and for identifying and treating at least one of Cachexia or Sarcopenia are disclosed. The system may include a dynamometer provided to patients, the dynamometer being configured to perform remote monitoring. The dynamometer may be used and data may be collected by patients without the supervision of a healthcare professional. Using the collected data, lean muscle mass may be tracked over time, to provide fast and more accurate assessment of conditions. Using such an assessment, early treatment options can be targeted to patients to avoid progression into various disease states, for examples, more severe states of at least one of Cachexia or Sarcopenia.
Owner:NXGEN MED LLC
Yeast cDNA library of anti-SVV (Seneca valley virus) VHH ((variable domain of heavy chain of heavy 2chain) antibody as well as construction method and application of yeast cDNA library
The invention discloses a yeast cDNA library of an anti-SVV (Seneca valley virus) VHH ((variable domain of heavy chain of heavy 2chain) antibody as well as a construction method and an application ofthe yeast cDNA library. Lymphocyte is obtained through separation in SVV inactivated vaccine immunized camel peripheral blood, a cDNA fragment of the anti-SVV VHH antibody is obtained with an RT-PCR (reverse transcription-polymerase chain reaction) method, the cDNA fragment and Pgadt7-Rec are used for co-transformation of yeast competent cells, and the cDNA library of the anti-SVV VHH antibody isobtained. Results show that the tilter of the library is about 7.2*10<6> cfu / ml, and the recombination rate of the library is 100%. Further preliminary functional identification on the library shows that the constructed yeast library has the anti-SVV VHH antibody. A platform is provided for screening of the anti-SVV VHH antibody, and a novel method is provided for early treatment and diagnosis ofSeneca valley.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
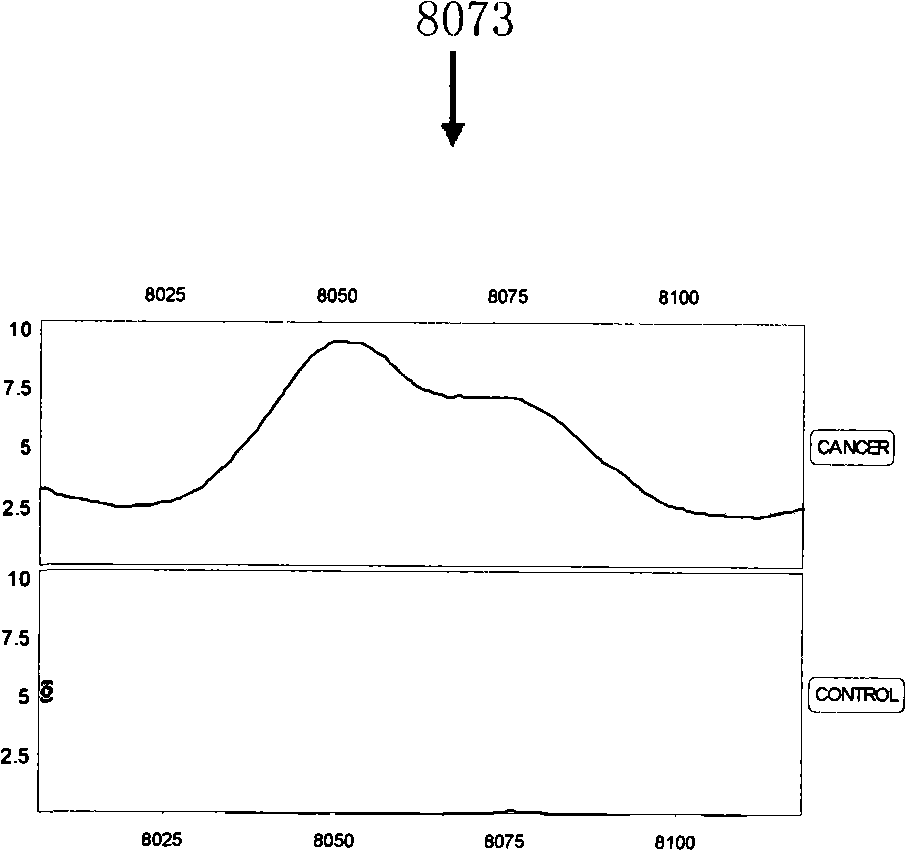
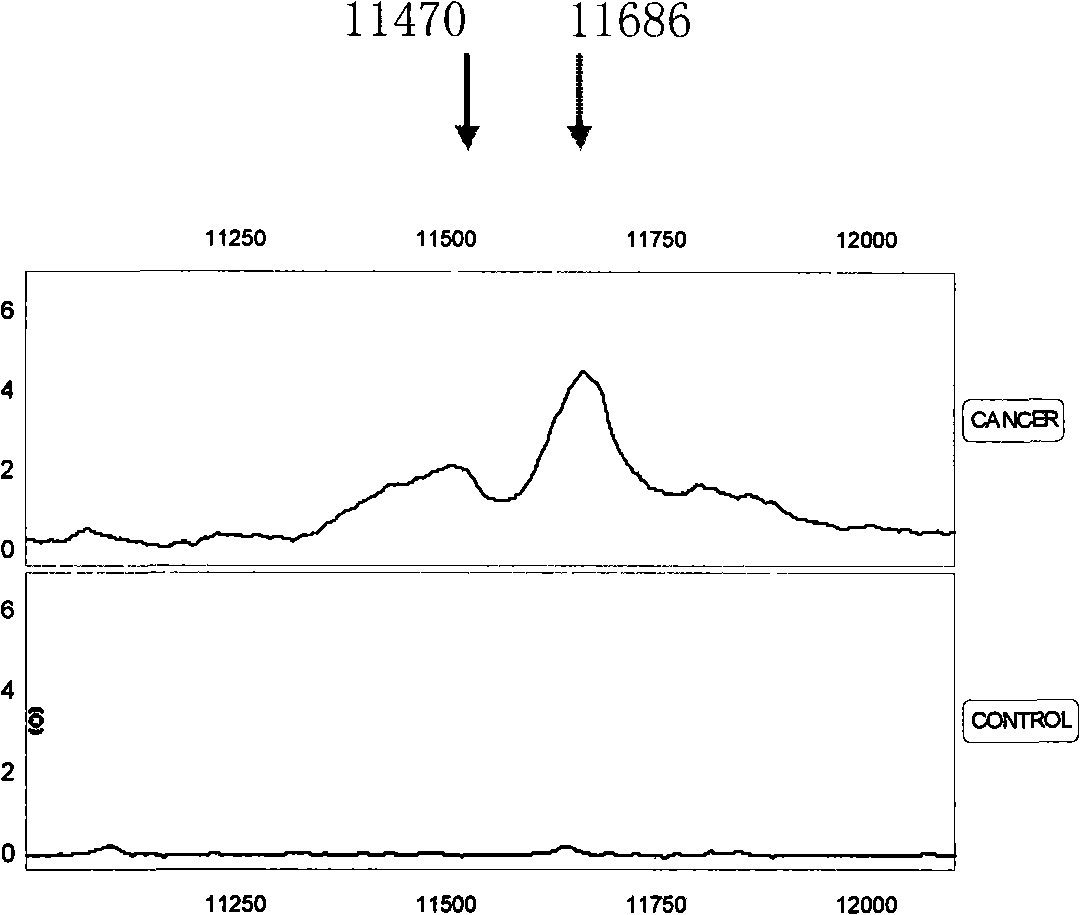
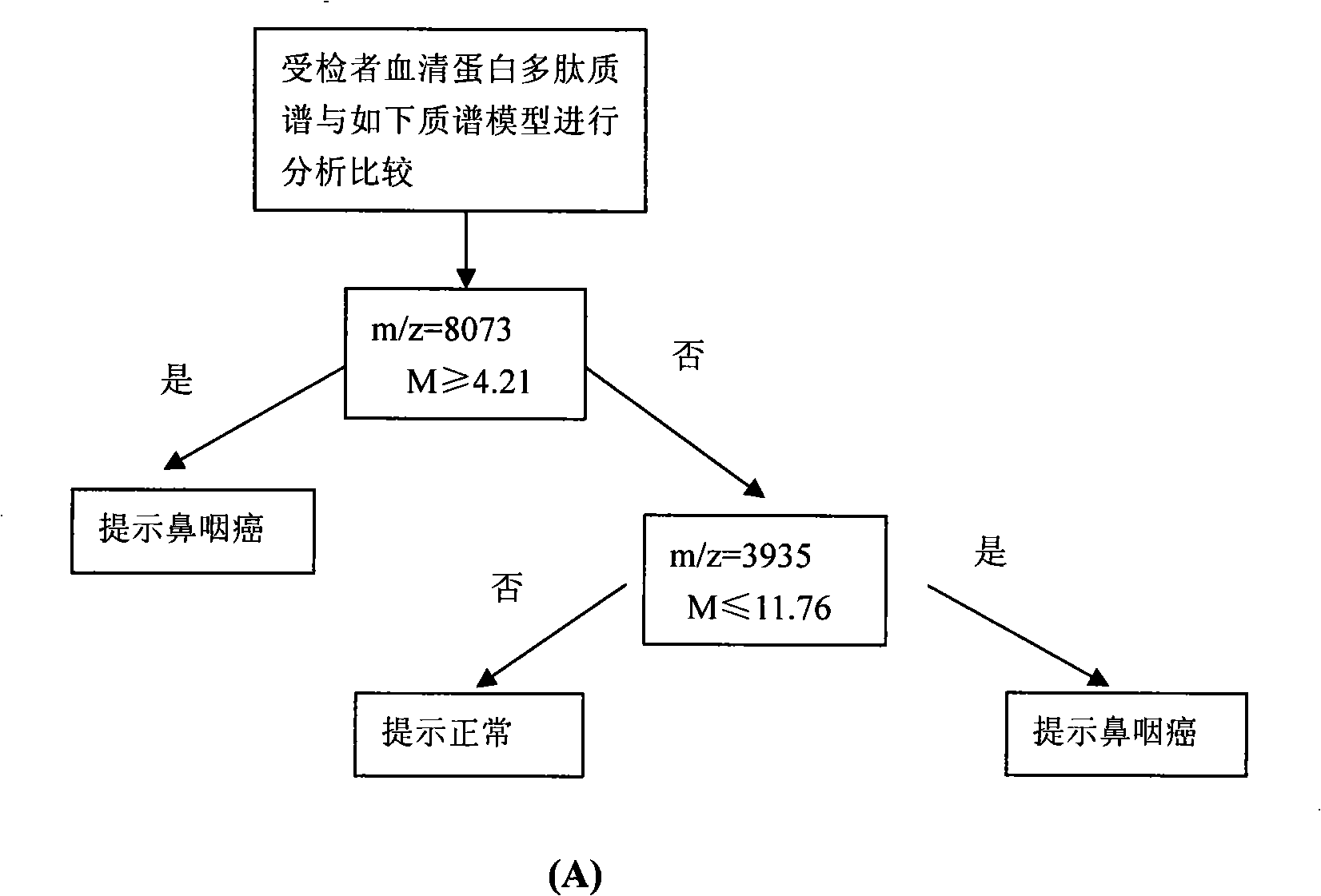
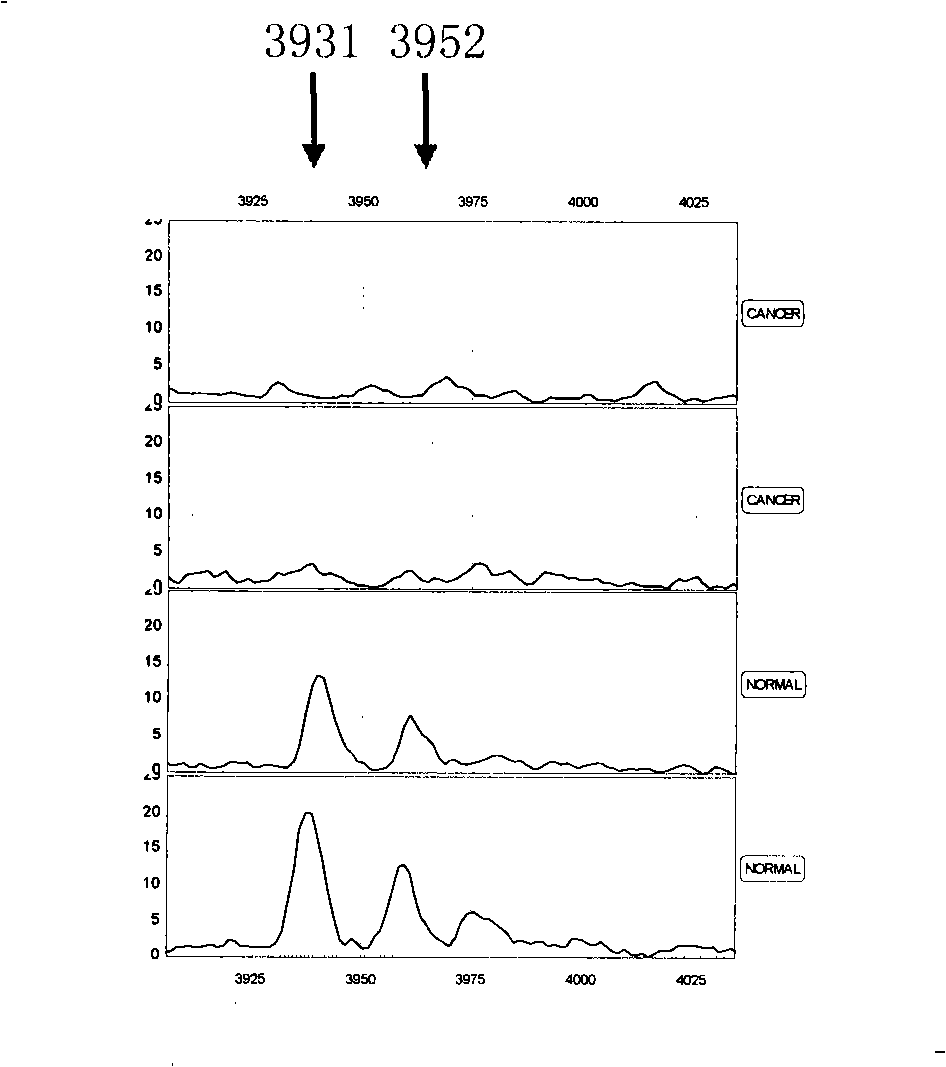
Optimizing mass spectrogram model for detecting nasopharyngeal cancer characteristic protein and preparation method and application thereof
InactiveCN101329350AIncreased sensitivityImprove featuresComponent separationBiological testingHigh risk populationsCase fatality rate
The invention relates to an optimum mass spectrometry model and a preparation method thereof for detecting the feature protein of nasopharyngeal cancer, belonging to the field of mass spectrometry detection technique. The invention is characterized in that six up-regulated proteins and five lower-regulated proteins are screened from the blood serum to be used as the feature proteins; any two or more proteins of the eleven proteins are chosen so as to establish a blood serum feature protein mass spectrometry model of identification with two in a group for patients with nasopharyngeal cancer and normal people, and patients with benign nasopharyngeal cancer disease, lymphatic metastasis of nasopharyngeal cancer and remote metastasis of nasopharyngeal cancer according to the mass-charge ratio m / z of each protein peak and the critical peak average value of the protein; the preparation method of the invention provides a foundation for discovering new nasopharyngeal cancer biological marks. The method of the invention is better than any single detection method adopted currently for the detection of the nasopharyngeal cancer, and provides a non-invasive technique for the early detection and early treatment of the nasopharyngeal cancer, thus providing a new method for reducing the mortality of the nasopharyngeal cancer, improving the cure rate of the nasopharyngeal cancer and screening and examining the nasopharyngeal cancer for high-risk population further.
Owner:许洋
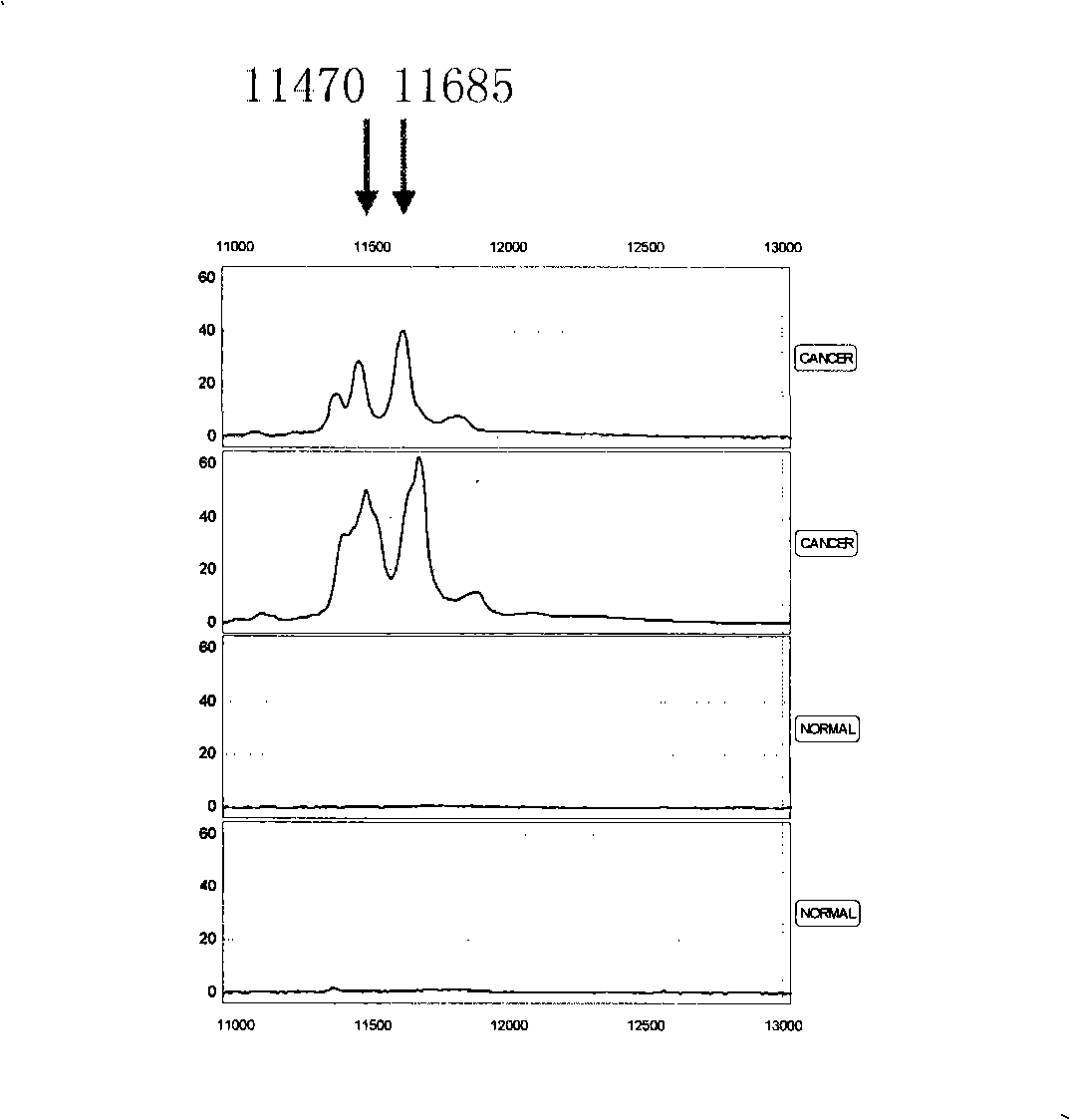
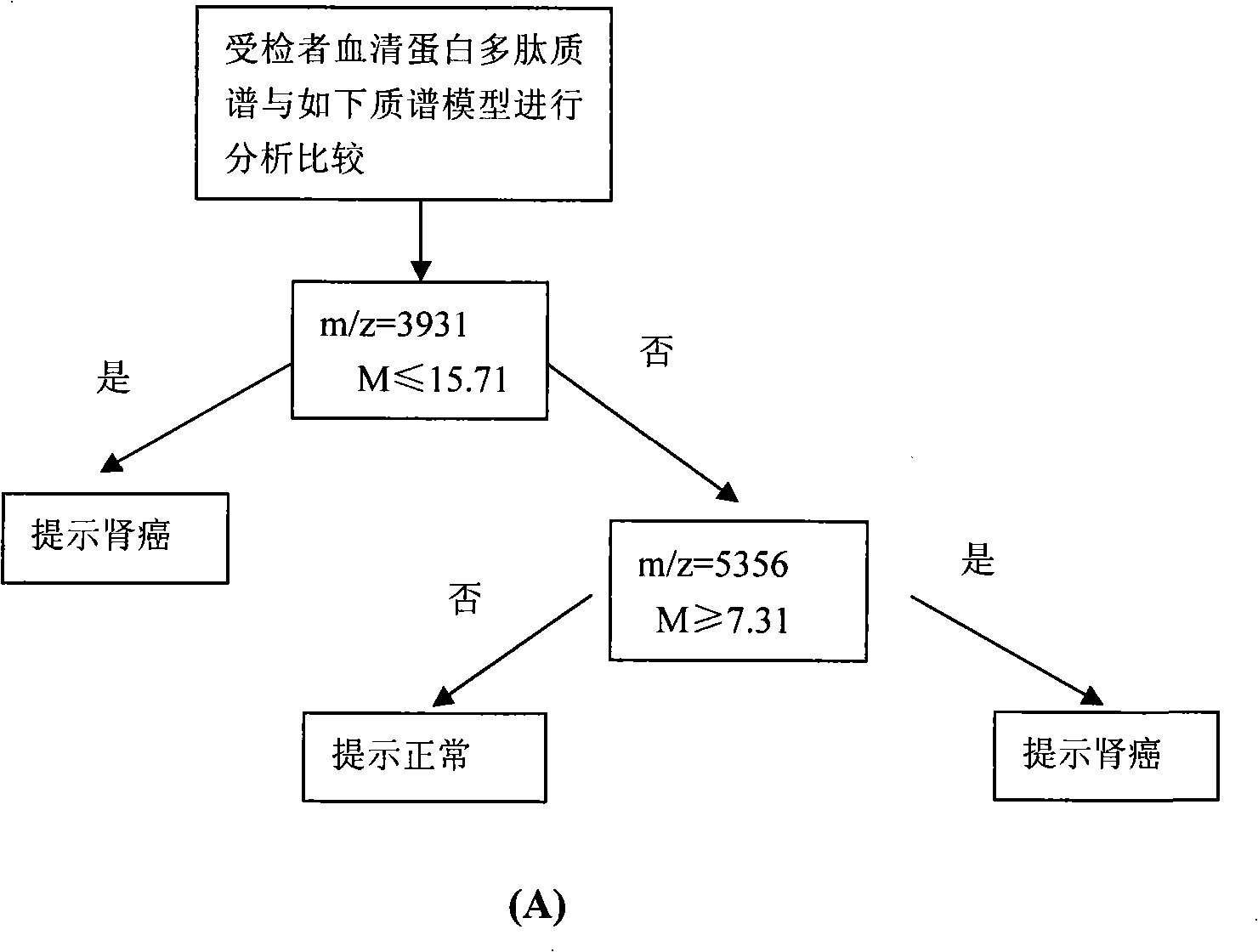
Optimizing mass spectrogram model for detecting kidney cancer characteristic protein and preparation method and application thereof
InactiveCN101329347AIncreased sensitivityImprove featuresComponent separationBiological testingHigh risk populationsCase fatality rate
The invention relates to an optimum mass spectrometry model and a preparation method thereof for detecting the feature protein of renal cancer, belonging to the field of mass spectrometry detection technique. The invention is characterized in that seven up-regulated proteins and three lower-regulated proteins are screened from the blood serum to be used as the feature proteins; any two or more proteins of the ten proteins are chosen so as to establish a blood serum feature protein mass spectrometry model of identification with two in a group for patients with renal cancer and normal people, and patients with benign renal cancer disease, lymphatic metastasis of renal cancer and remote metastasis of renal cancer according to the mass-charge ratio m / z of each protein peak and the critical peak average value of the protein; the preparation method of the invention provides a foundation for discovering new renal cancer biological marks. The method of the invention is better than any single detection method adopted currently for the detection of the renal cancer, and provides a non-invasive technique for the early detection and early treatment of the renal cancer, thus providing a new method for reducing the mortality of the renal cancer, improving the cure rate of the renal cancer and screening and examining the renal cancer for high-risk population further.
Owner:许洋
Primer pair, probe and kit for detecting African swine fever virus and application of primer pair, probe and kit
InactiveCN113215328AHigh detection sensitivityShort detection timeMicrobiological testing/measurementMicroorganism based processesAfrican swine feverViral nucleic acid
The invention discloses a primer pair, a probe and a kit for detecting African swine fever virus. The kit comprises the primer pair and the probe which are designed for an African swine fever virus p72 gene sequence shown in SEQ ID NO: 1 or a part of the sequence. The kit for detecting the African swine fever virus is based on a nested RPA detection technology (nestRPA), can detect 1 copy / microliter ASFV virus nucleic acid fragments at least, has ultrahigh sensitivity, does not need expensive detection equipment, and is short in detection time which only needs 12min. The technology can early discover African swine fever, provides a new technical support for early isolation and early treatment, and has a very good application prospect.
Owner:黄婉秋 +2
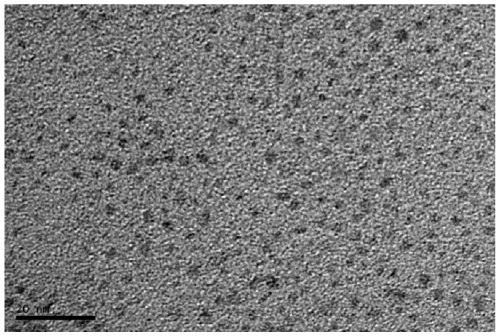
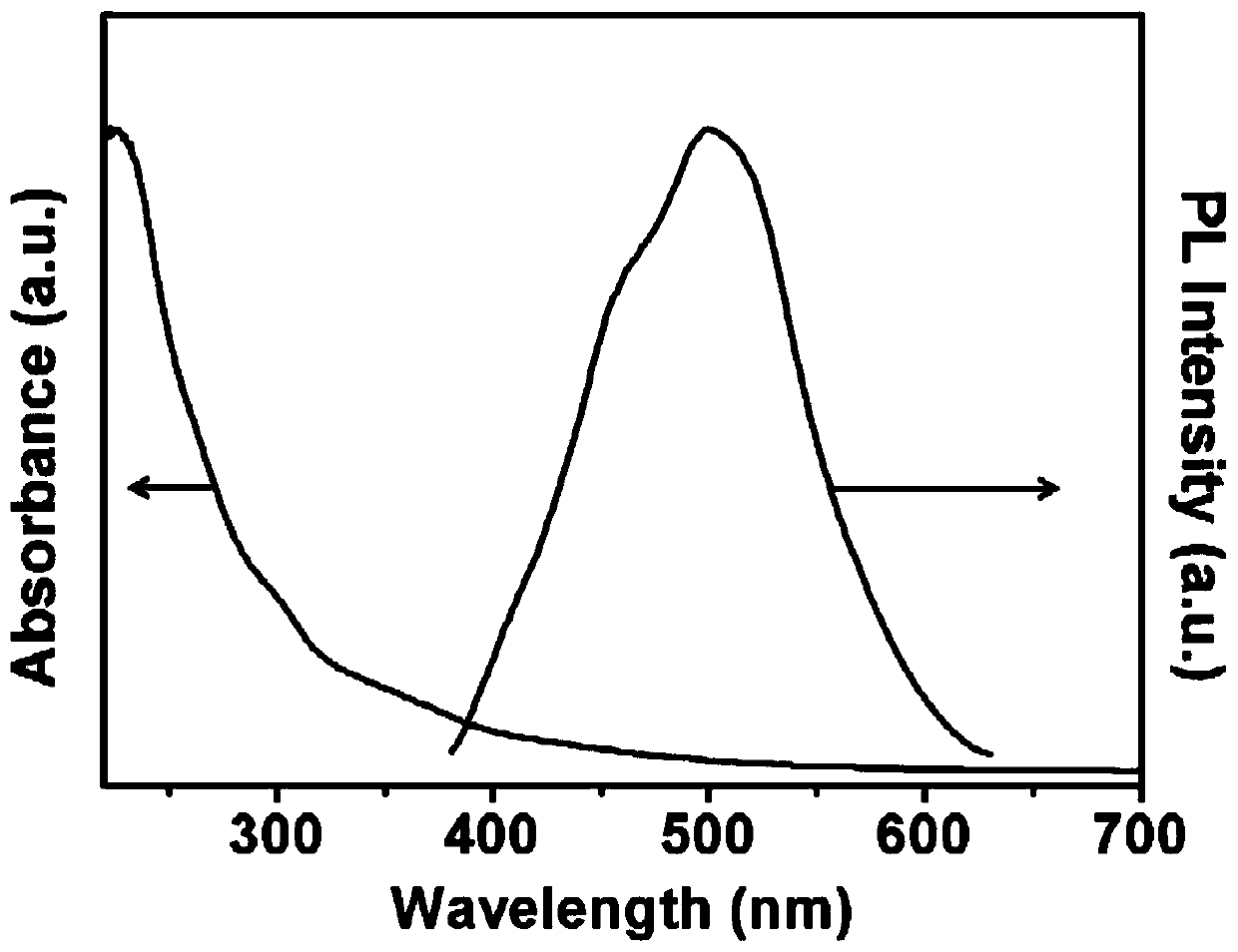
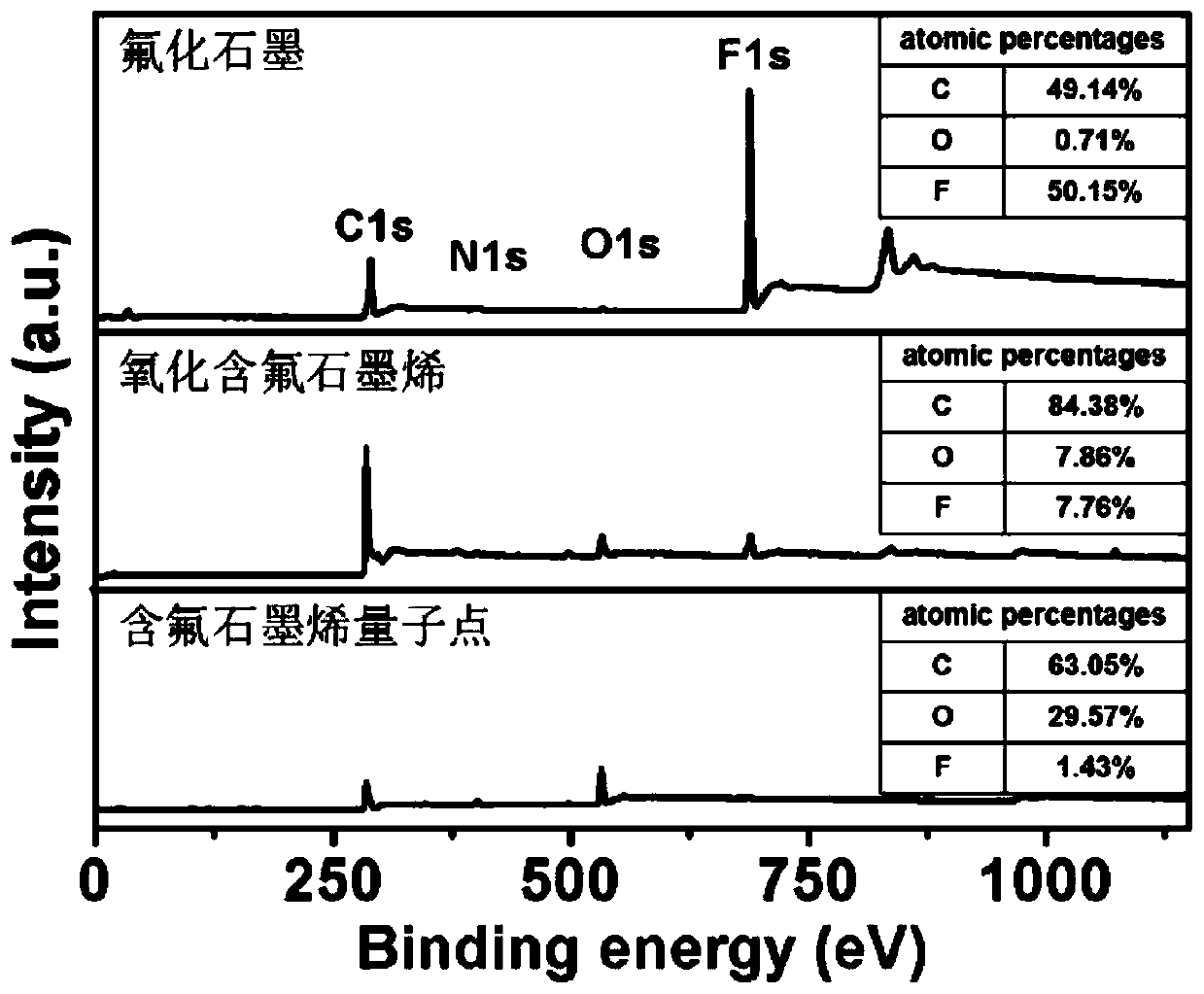
Fluorine-containing graphene quantum dot, preparation and application thereof as photodynamic therapy photosensitizer
ActiveCN111518552ASmall sizeUniform shapeMaterial nanotechnologyPhotodynamic therapyFluorographeneSinglet oxygen
The invention discloses a fluorine-containing graphene quantum dot, a preparation method and application thereof as a photodynamic therapy photosensitizer, and belongs to the field of biomedical materials. The average thickness of the prepared fluorine-containing graphene quantum dot is 1.0-3.0 nanometers, the size is 2.0-3.0 nanometers, the fluorine content is 1%-2%, the oxygen content is 20%-30%, and the carbon content is 60%-70%. The fluorine-containing graphene quantum dot is smaller in size, uniform in morphology, stable in structure, high in singlet oxygen yield under visible light irradiation, extremely low in cytotoxicity, good in water solubility and more excellent in biocompatibility and is an ideal photosensitizer for photodynamic therapy and is suitable for the early treatmentprocess of esophagus cancer, skin cancer, lung cancer and the like and has a wide application prospect.
Owner:ANHUI UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com