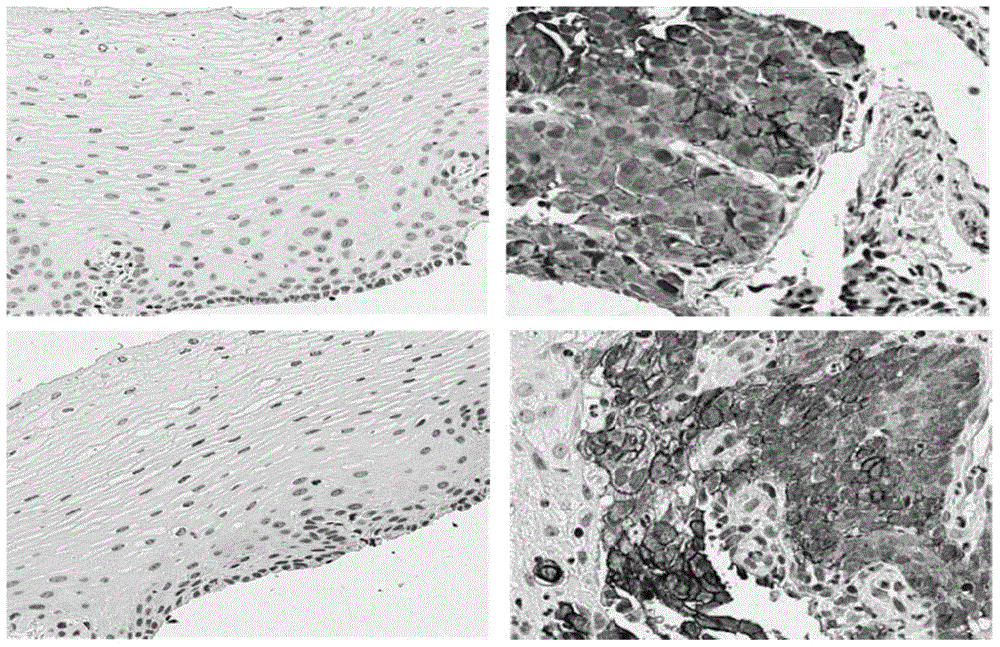
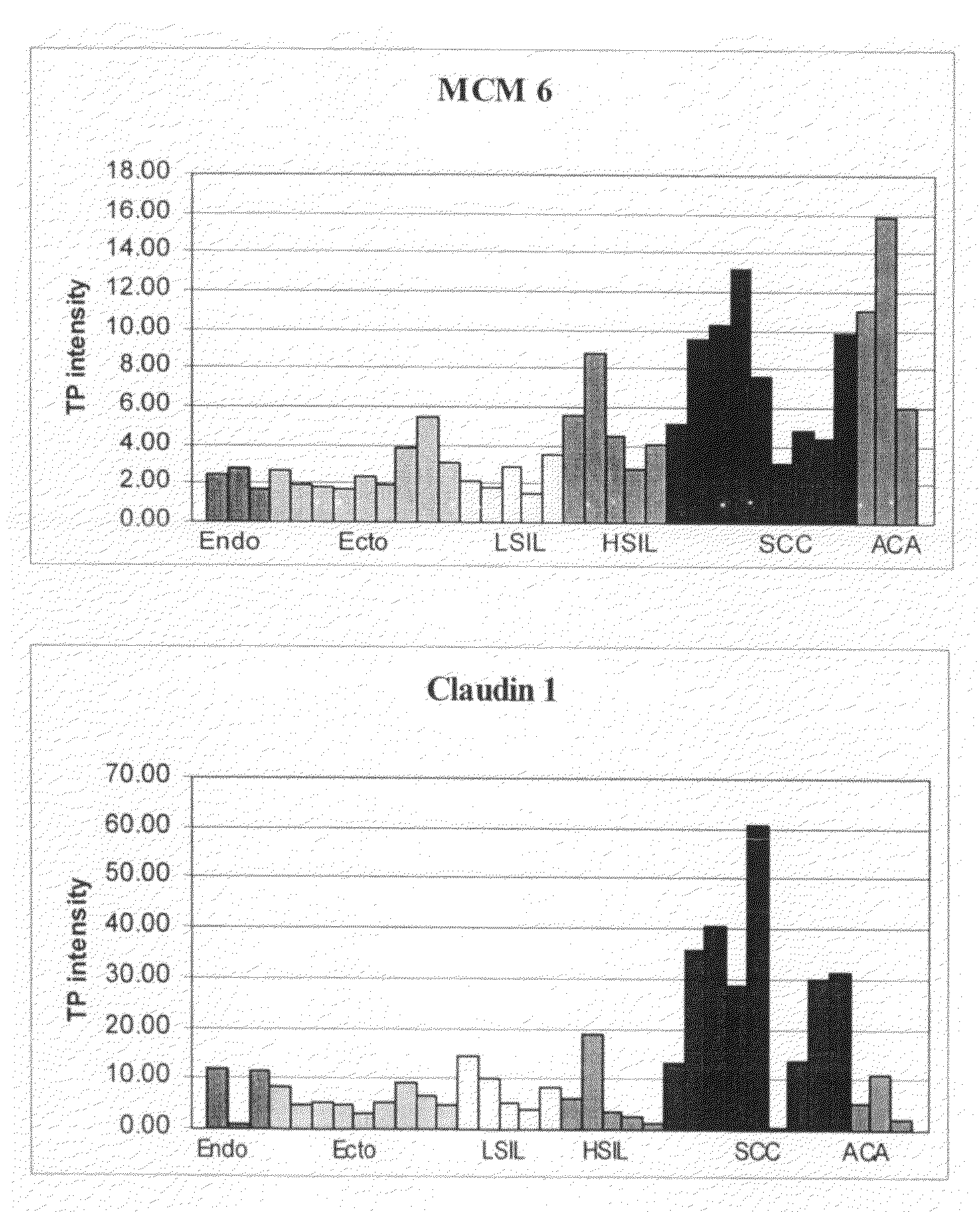
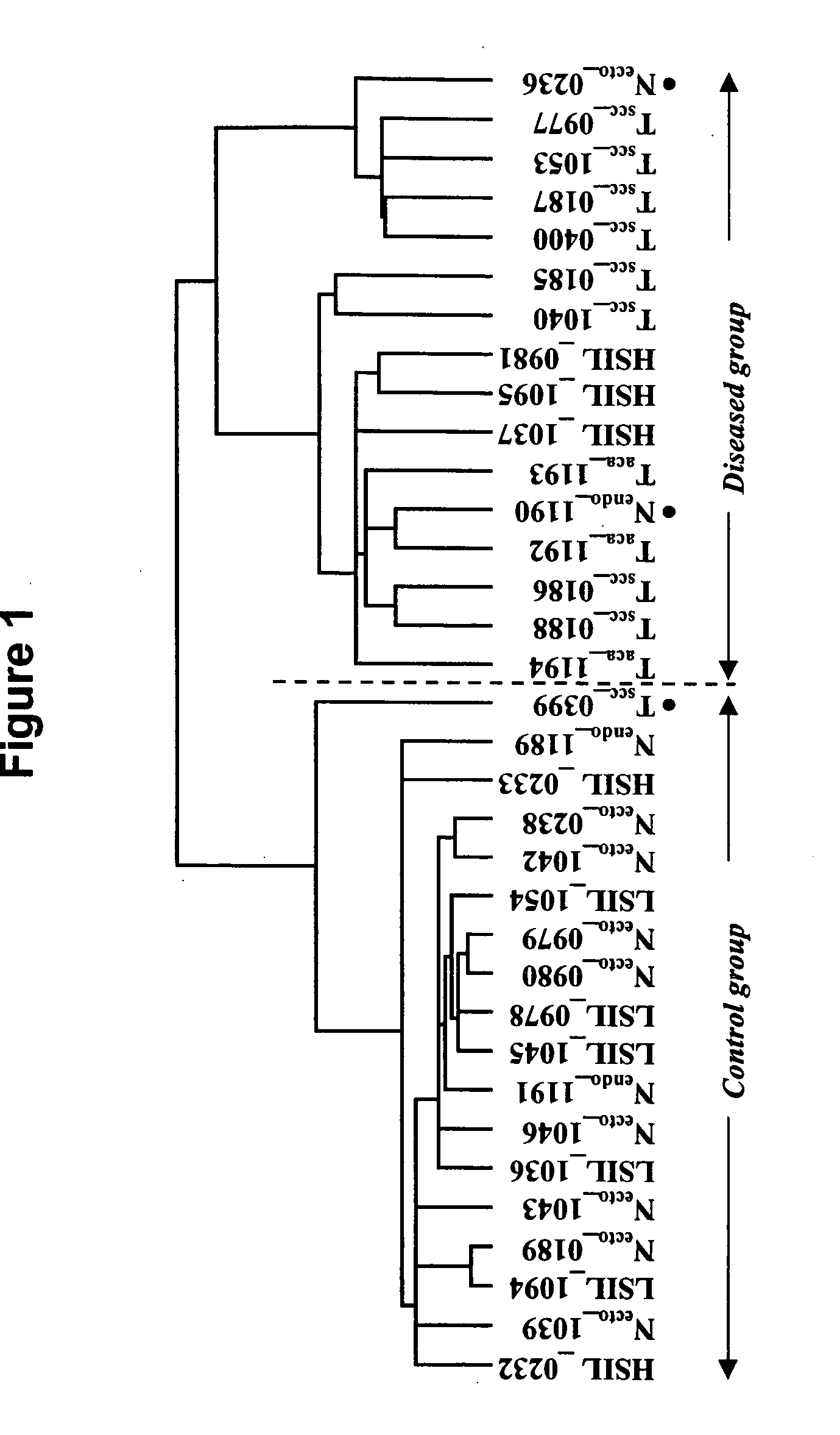
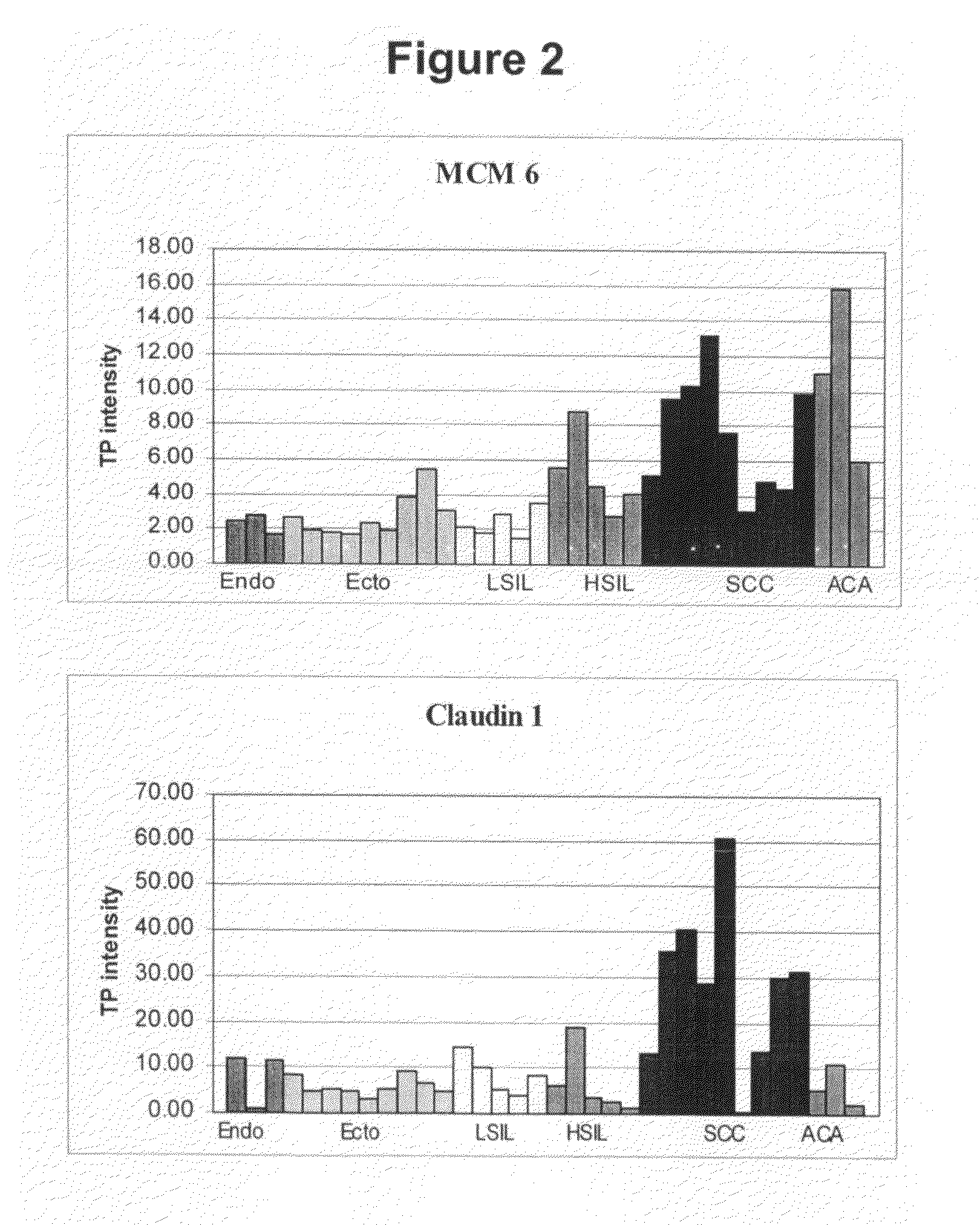
Patents
Literature
149 results about "Precancerous condition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A precancerous condition is a condition or lesion involving abnormal cells which are associated with an increased risk of developing into cancer. Clinically, precancerous conditions encompass a variety of conditions or lesions with an increased risk of developing into cancer. Some of the most common precancerous conditions include certain colon polyps, which can progress into colon cancer, monoclonal gammopathy of undetermined significance, which can progress into multiple myeloma or myelodysplastic syndrome. and cervical dysplasia, which can progress into cervical cancer. Pathologically, precancerous lesions can range from benign neoplasias, which are tumors which do not invade neighboring normal tissues or spread to distant organs, to dysplasia, which involves collections of abnormal cells which in some cases have an increased risk of progressing to anaplasia and invasive cancer. Sometimes the term "precancer" is also used for carcinoma in situ, which is a noninvasive cancer that has not progressed to an aggressive, invasive stage. As with other precancerous conditions, not all carcinoma in situ will progress to invasive disease.
Electrical bioimpedance analysis as a biomarker of breast density and/or breast cancer risk
InactiveUS20090171236A1Ultrasonic/sonic/infrasonic diagnosticsDiagnostic recording/measuringBiologic markerBreast density
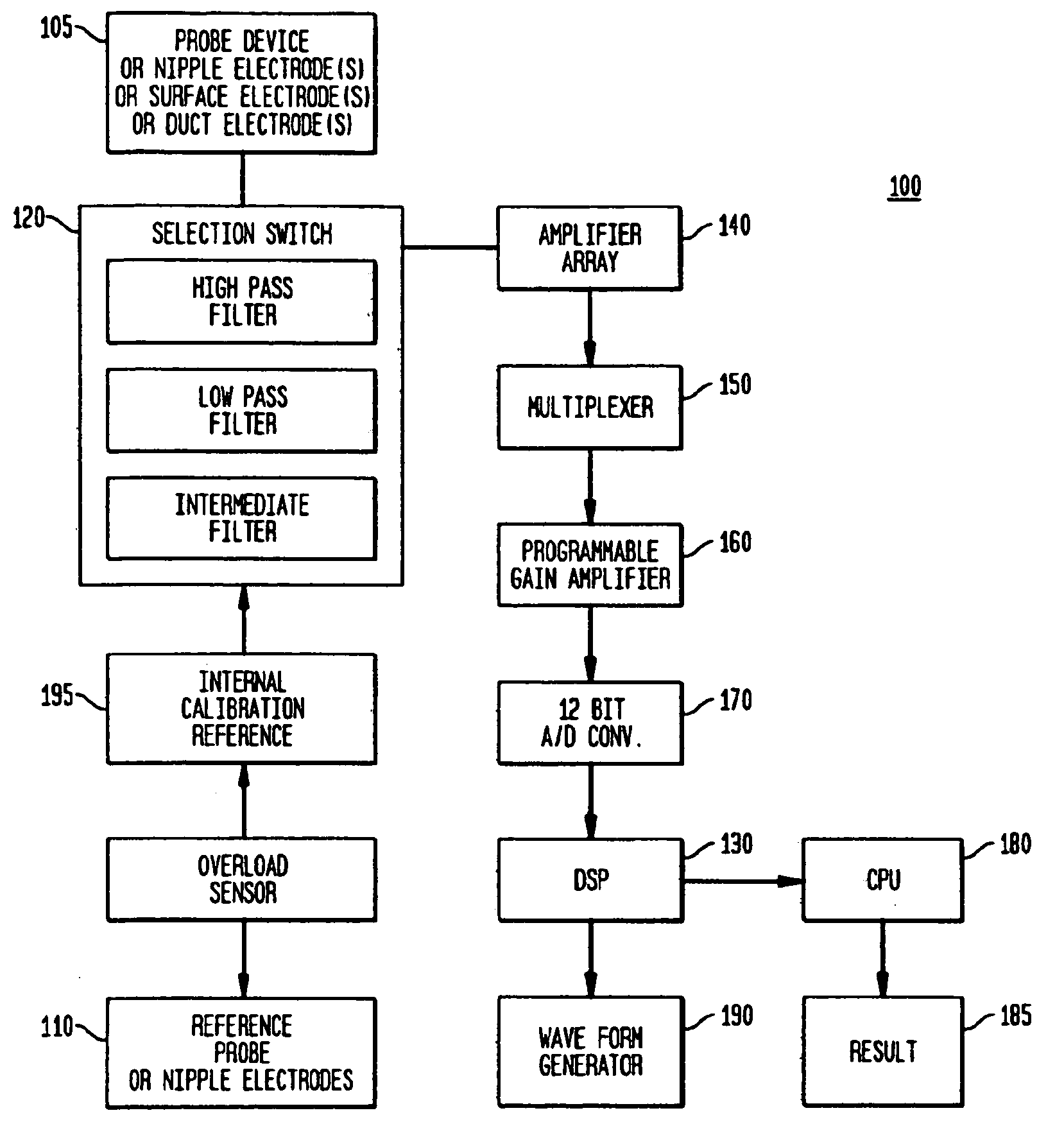
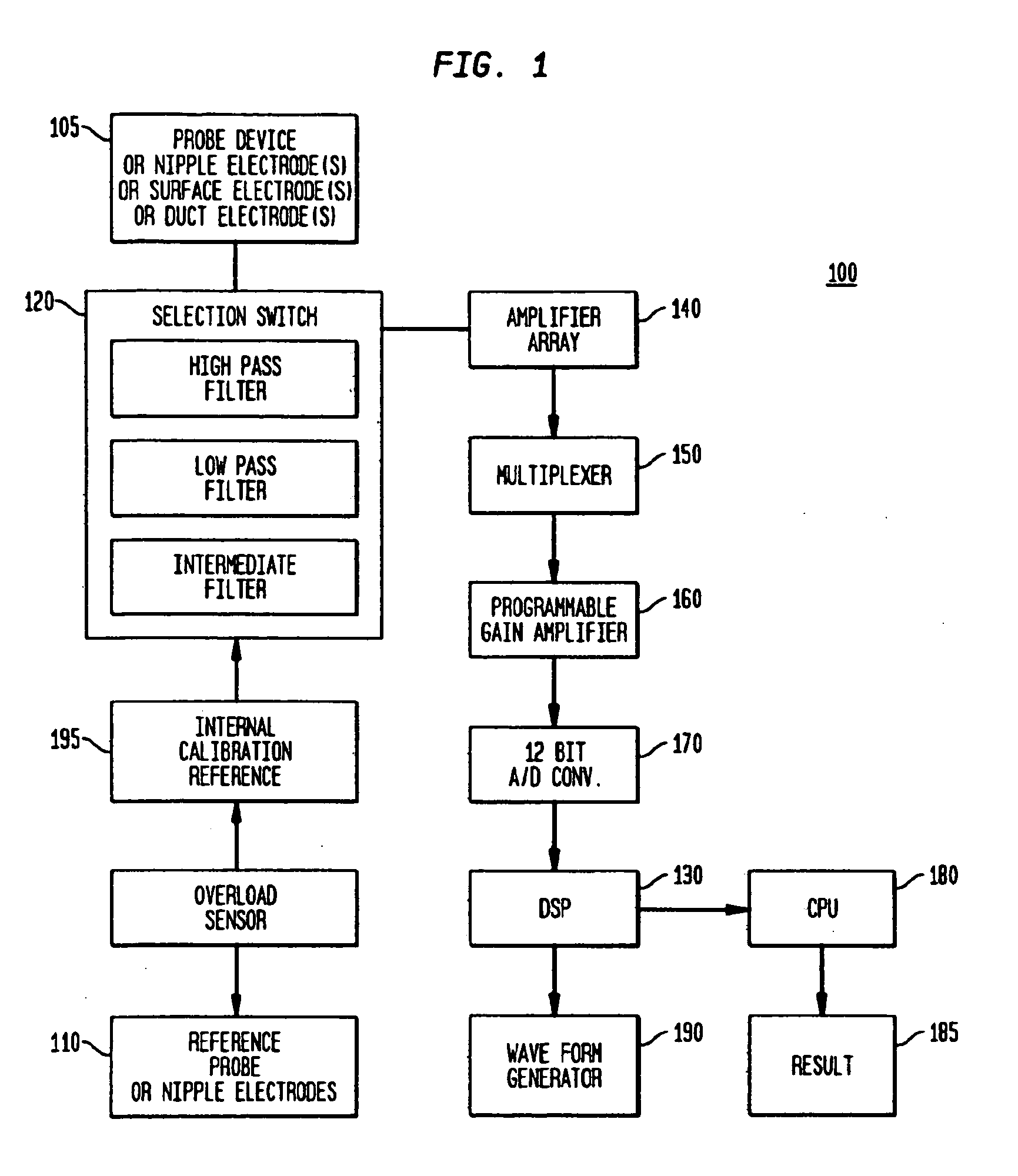
Methods and systems are provided for the noninvasive measurement of the subepithelial impedance of the breast and for assessing the risk that a substantially asymptomatic female patient will develop or be at substantially increased risk of developing proliferative or pre-cancerous changes in the breast, or may be at subsequent risk for the development of pre-cancerous or cancerous changes. A plurality of electrodes are used to measure subepithelial impedance of parenchymal breast tissue of a patient at one or more locations and at least one frequency, particularly moderately high frequencies. The risk of developing breast cancer is assessed according to measured and expected or estimated values of subepithelial impedance for the patient and according to one or more experienced-based algorithms. Devices for practicing the disclosed methods are also provided.
Owner:EPI SCI LLC
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176258A1Improve efficiencyIncrease resourcesBiological material analysisDepsipeptidesImmunodominant EpitopesMonoclonal antibody
Disclosed herein among other MN / CA IX-related inventions are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Subsets of the new antibodies are to either the proteoglycan-like (PG) domain or to the carbonic anhydrase (CA) domain of MN / CA IX, and methods are provided by which antibodies can be prepared to the other MN / CA IX domains. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Methods for cancer imaging
InactiveUS6989140B2Ultrasonic/sonic/infrasonic diagnosticsIn-vivo radioactive preparationsCancer cellFluorescence
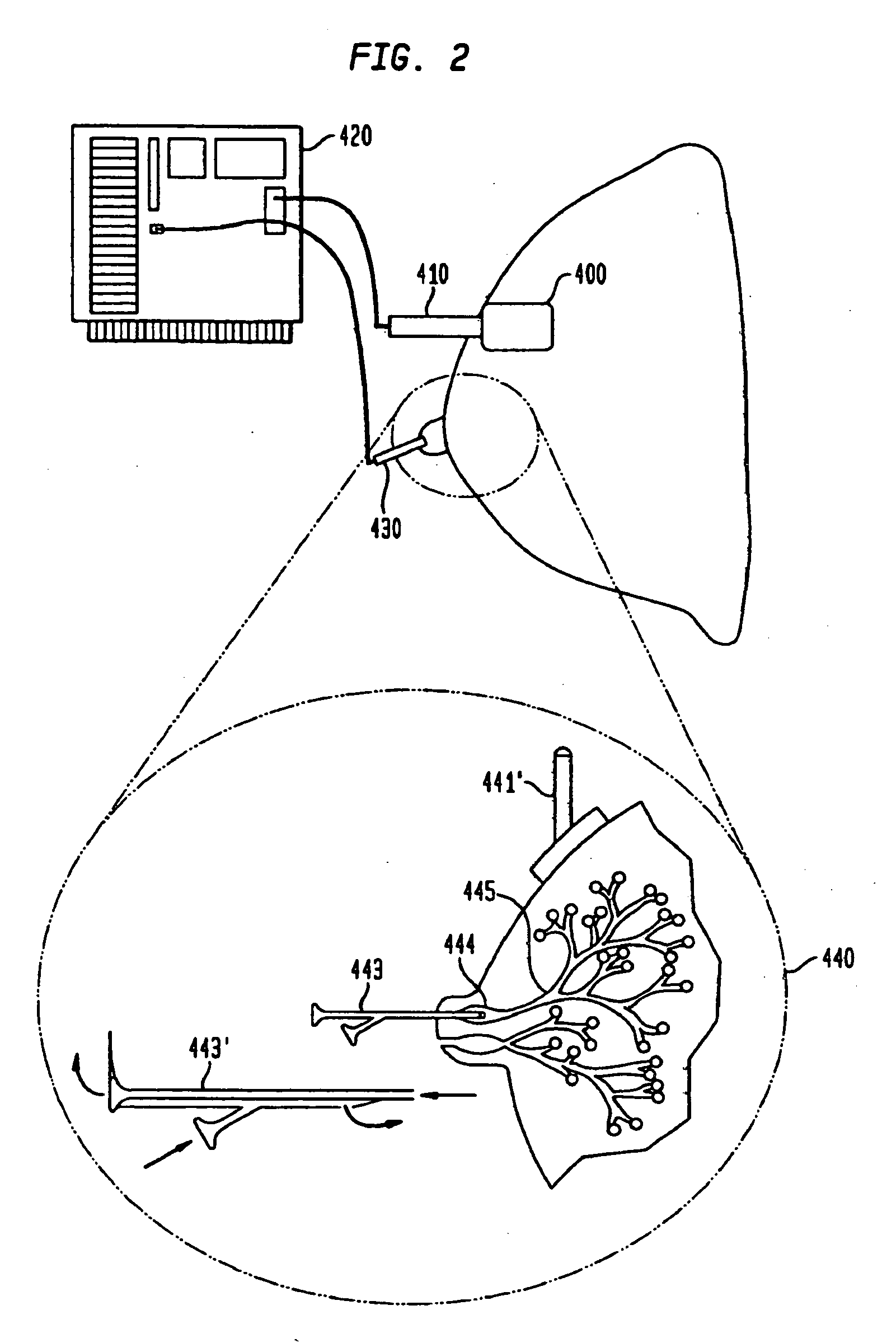
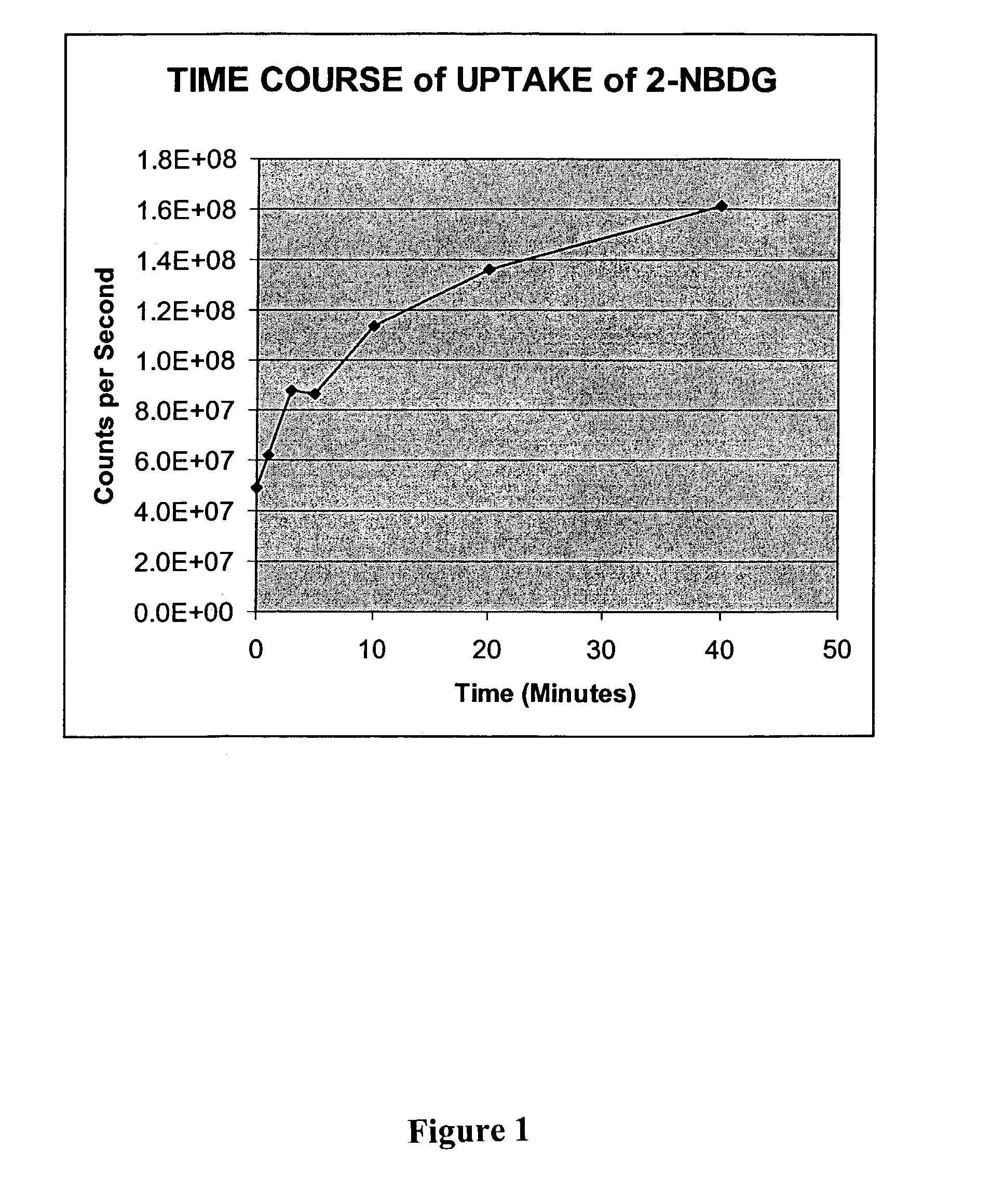
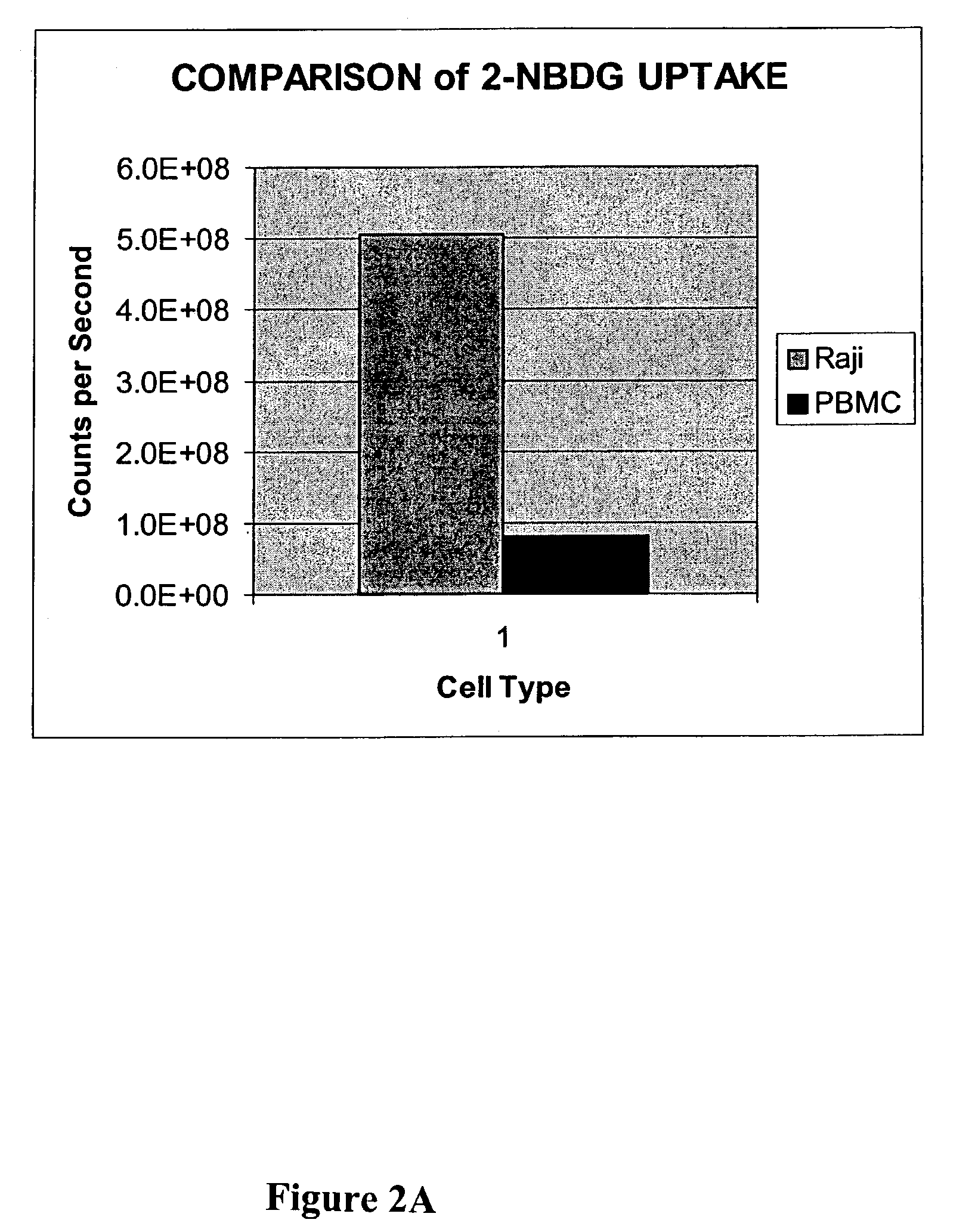
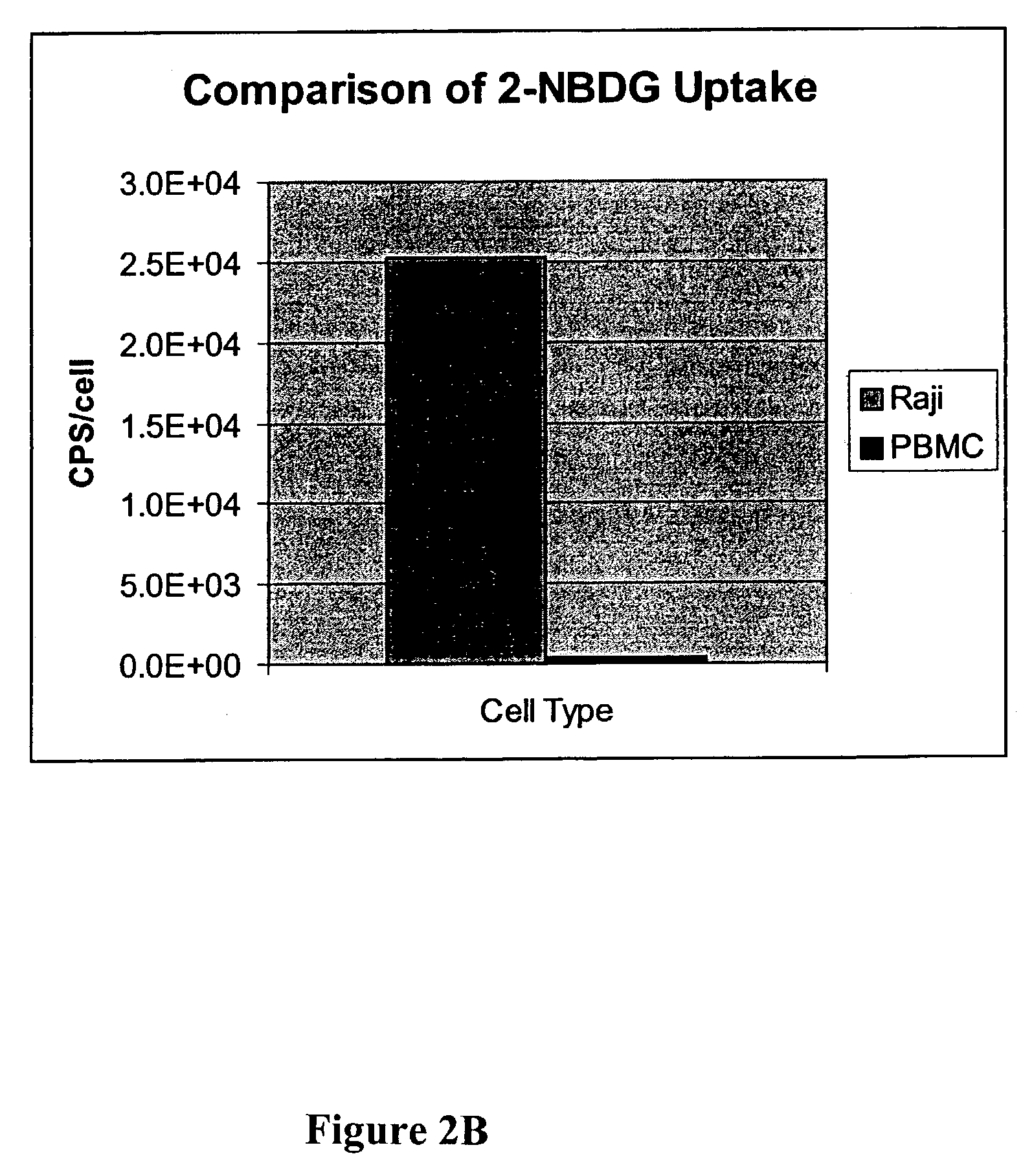
Methods are provided for cancer and pre-cancer detection by increased uptake of fluorophore glucose or deoxyglucose conjugates in cancerous and pre-cancerous cells relative to normal cells.
Owner:THRESHOLD PHARM INC
Soluble form of carbonic anhydrase IX (S-CA IX), assays to detect s-CA IX, CA IX'S coexpression with HER-2/NEU/C-ERBB-2, and CA IX-specific monoclonal antibodies to non-immunodominant epitopes
InactiveUS7816493B2Good curative effectSugar derivativesBiological material analysisKilodaltonC erbb 2
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) found in body fluids, such as, urine and serum. Soluble CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons. Diagnostic / prognostic methods for precancer / cancer that detect or detect and quantitate s-CA IX in body fluids, are described. Also disclosed is the coexpression of CA IX and HER-2 that provides potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer / cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, useful diagnostically / prognostically and therapeutically for cancer / precancer. Preferred are new antibodies, specific for non-immunodominant epitopes of MN / CA IX, useful to detect soluble CA IX (s-CA IX) in body fluids, preferably in combination with antibodies specific to immunodominant epitopes of MN / CA IX.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Product comprising a nicotine-containing material and an Anti-cancer agent
InactiveUS20140088045A1Prevention reductionReduce riskSmall article dispensingOrganic active ingredientsTransdermal patchAnticarcinogen
The present invention provides a composition comprising a nicotine-containing material and an anti-cancer agent usable in the treatment and / or prevention or reduction of the risk of cancer and precancerous conditions as well as for preventing or reducing the risk of cancer recurrence. Furthermore, a composition comprising a nicotine-containing material and an anti-inflammatory agent usable in the treatment and / or prevention or reduction of the risk of inflammation, is provided. The nicotine containing composition can also include both an anti-cancer agent and an anti-inflammatory agent A device for administering the composition of the present invention to subjects can be a cigarette, smoking pipe, smokeless tobacco, electronic cigarette, transdermal patch or the like.
Owner:RIGAS BASIL +1
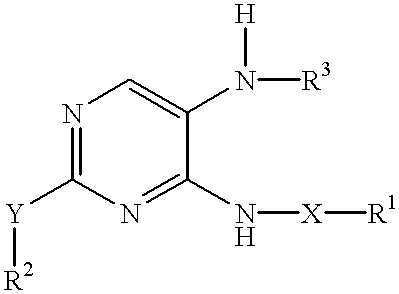
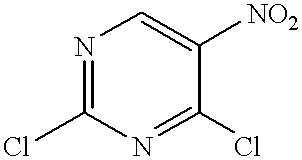
Method of inhibiting neoplastic cells with 4,5-diaminopyrimidine derivatives
InactiveUS6380206B1Eliminating and inhibiting growthModulating apoptosisOrganic active ingredientsBiocideDiaminopyrimidineOncology
A method for inhibiting neoplasia, particularly cancerous and precancerous lesions by exposing the affected cells to 4,5-diaminopyrimidine derivatives.
Owner:OSI PHARMA INC
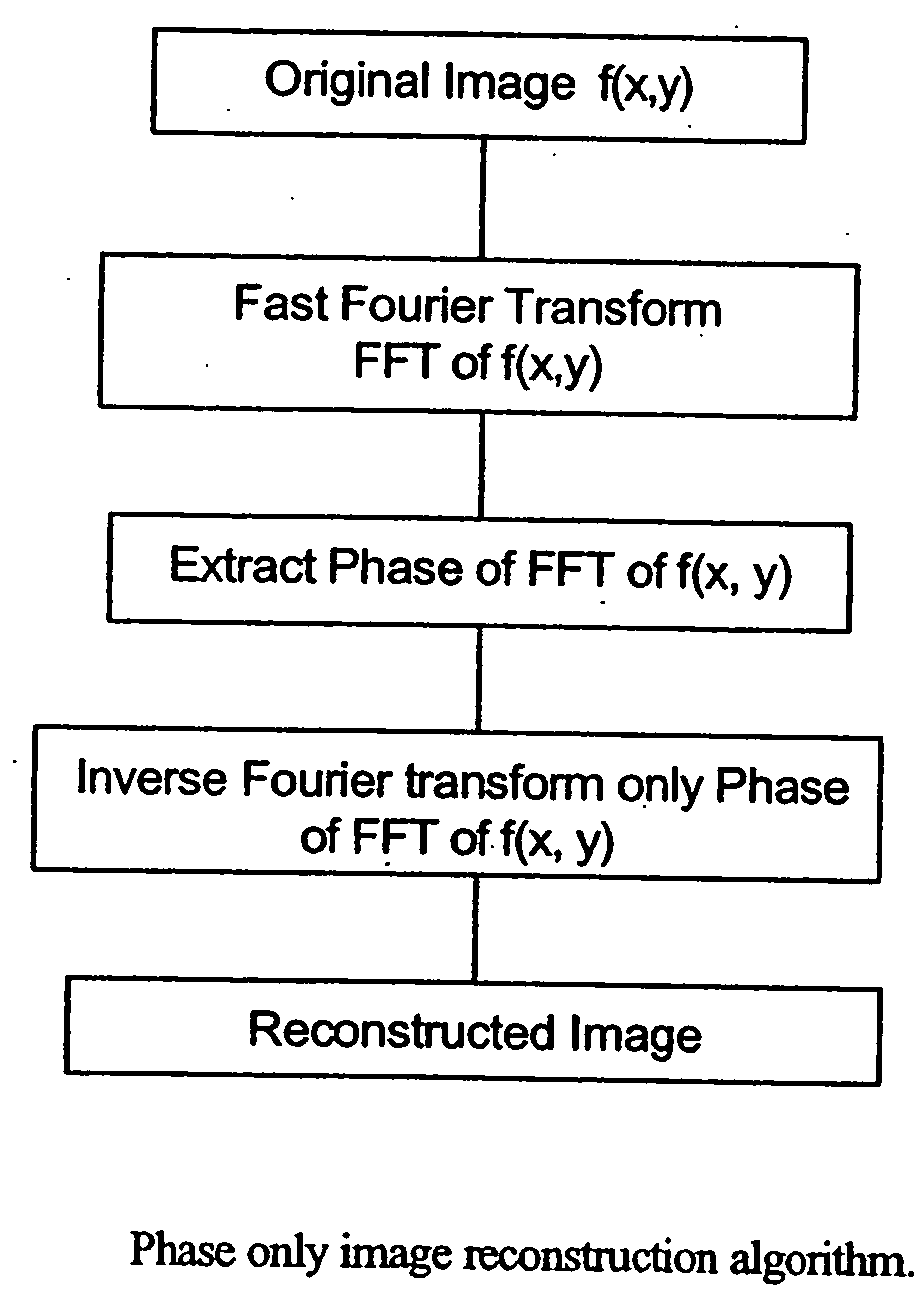
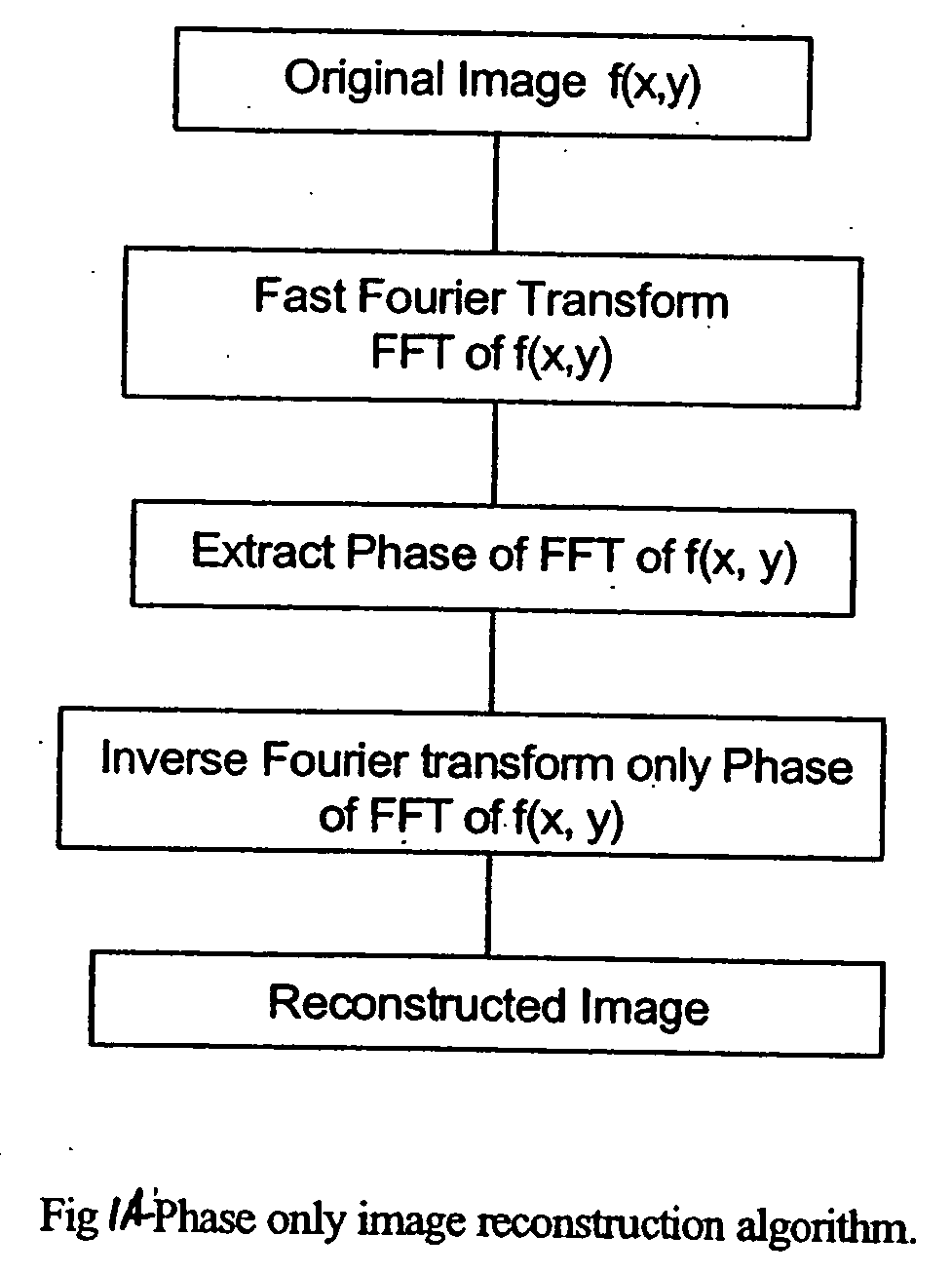
Phase based digital imaging
InactiveUS20060291707A1Enhanced diagnostic imageHigh frequency responseImage enhancementImage analysisDigital imagingMedical imaging
The present invention relates to systems and methods for medical imaging. Digital images are processed to provide phase images of a region of interest to aid in the diagnosis and treatment of various conditions. A preferred embodiment of the invention provides improved mammography screening for cancerous or precancerous conditions.
Owner:UNIV OF MASSACHUSETTS
Novel genes, compositions, kits, and methods for identification, assessment, prevention, and therapy of prostate cancer
InactiveUS20050191673A1Reduced expression levelImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureOncologyNovel gene
The invention relates to newly discovered nucleic acid molecules and proteins associated with prostate cancer including pre-malignant conditions. Compositions, kits, and methods for detecting, characterizing, preventing, and treating human prostate cancers are provided.
Owner:MILLENNIUM PHARMA INC
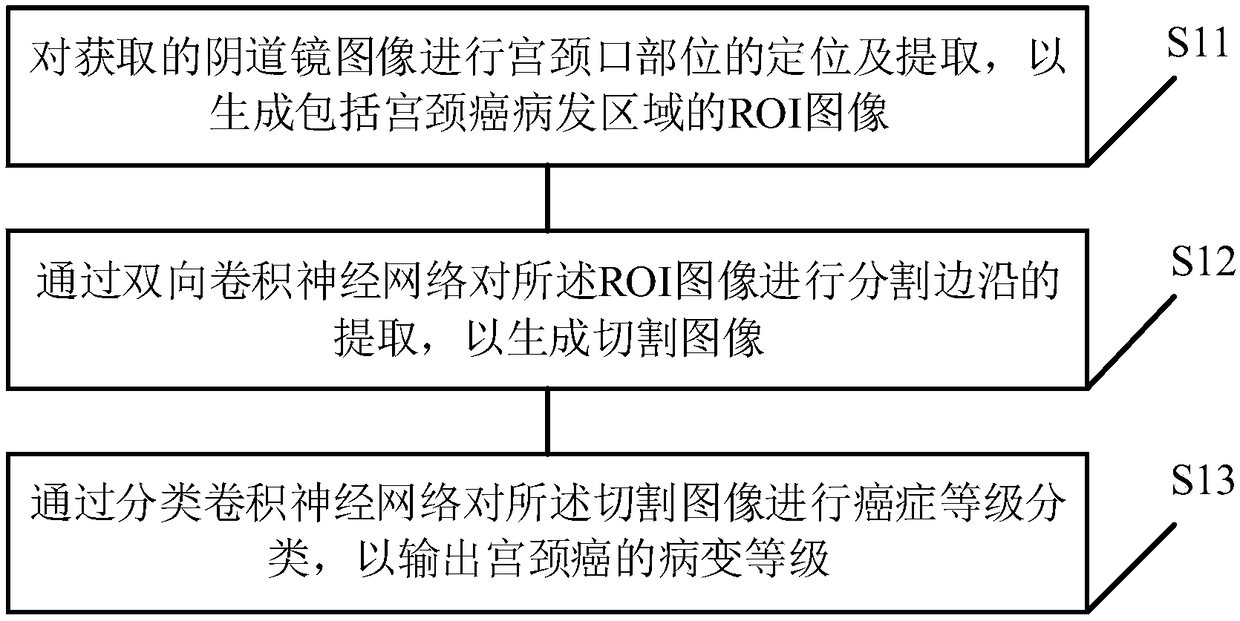
Colposcopy image-based cervical cancer detection method, device and equipment and medium
InactiveCN108510482AReduce constraintsSegmentation is fast and accurateImage enhancementImage analysisDisease areaNerve network
The invention discloses a colposcopy image-based cervical cancer detection method, device and terminal equipment and a computer readable storage medium. The method comprises the following steps of: ina bidirectional convolutional neural network-based cervical cancer detection model, positioning and extracting a cervical opening position of an obtained colposcopy image so as to generate an ROI image comprising a cervical cancer disease area; extracting a cutting edge of the ROI image through a bidirectional convolutional neural network so as to generate a cut image; and carrying out cancer grade classification on the cut image through a classification convolutional neural network so as to output a cervical cancer lesion grade and then rapidly and correctly obtain a cervical cancer detection result. According to the method, doctors that are lack of experiences can be helped to rapidly judge diseased regions, discover atypical diseased regions and judge the disease degrees and sampling regions, so that help and auxo-action are provided for discovering cervical cancer and precancerous lesions.
Owner:姚书忠 +3
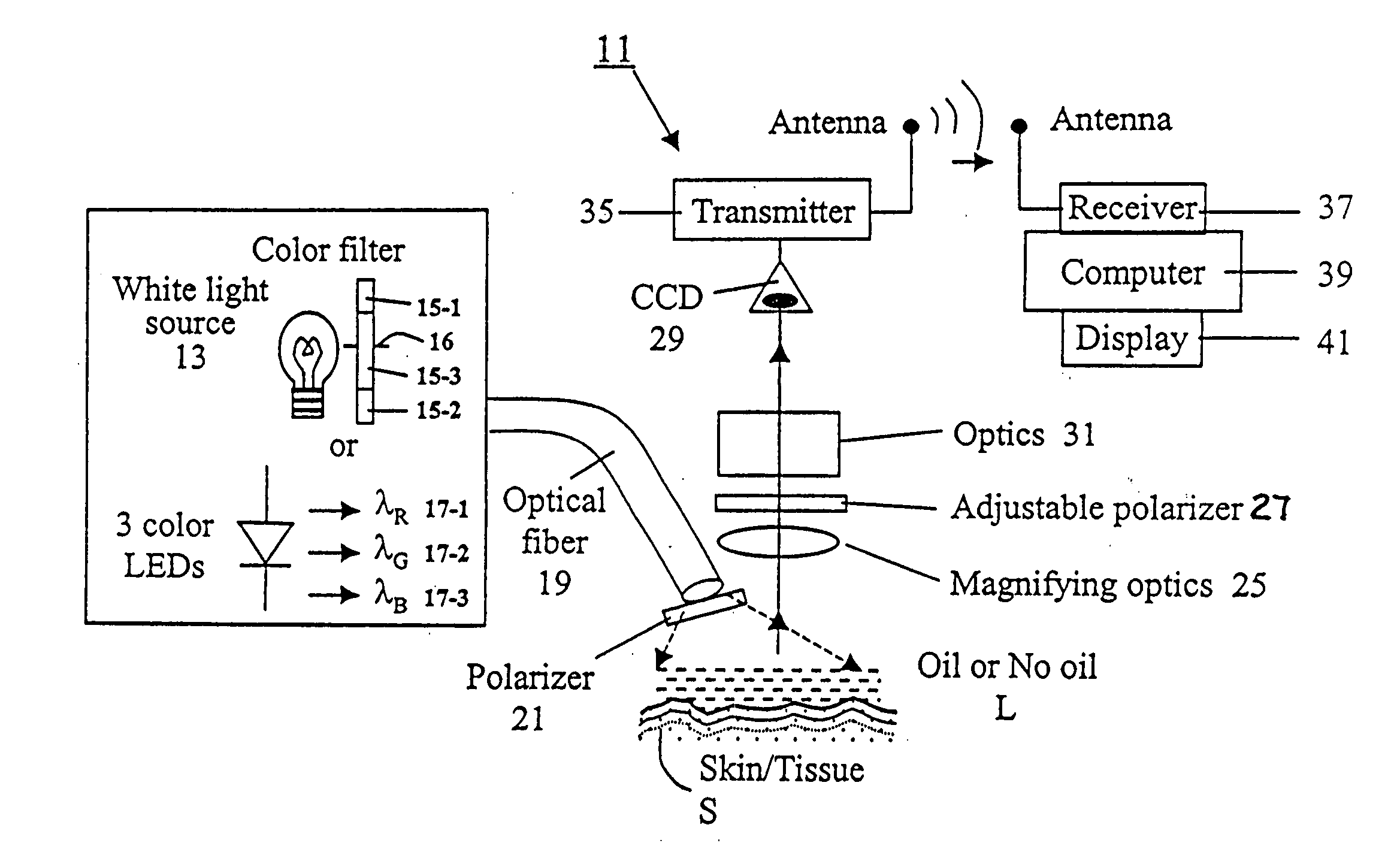
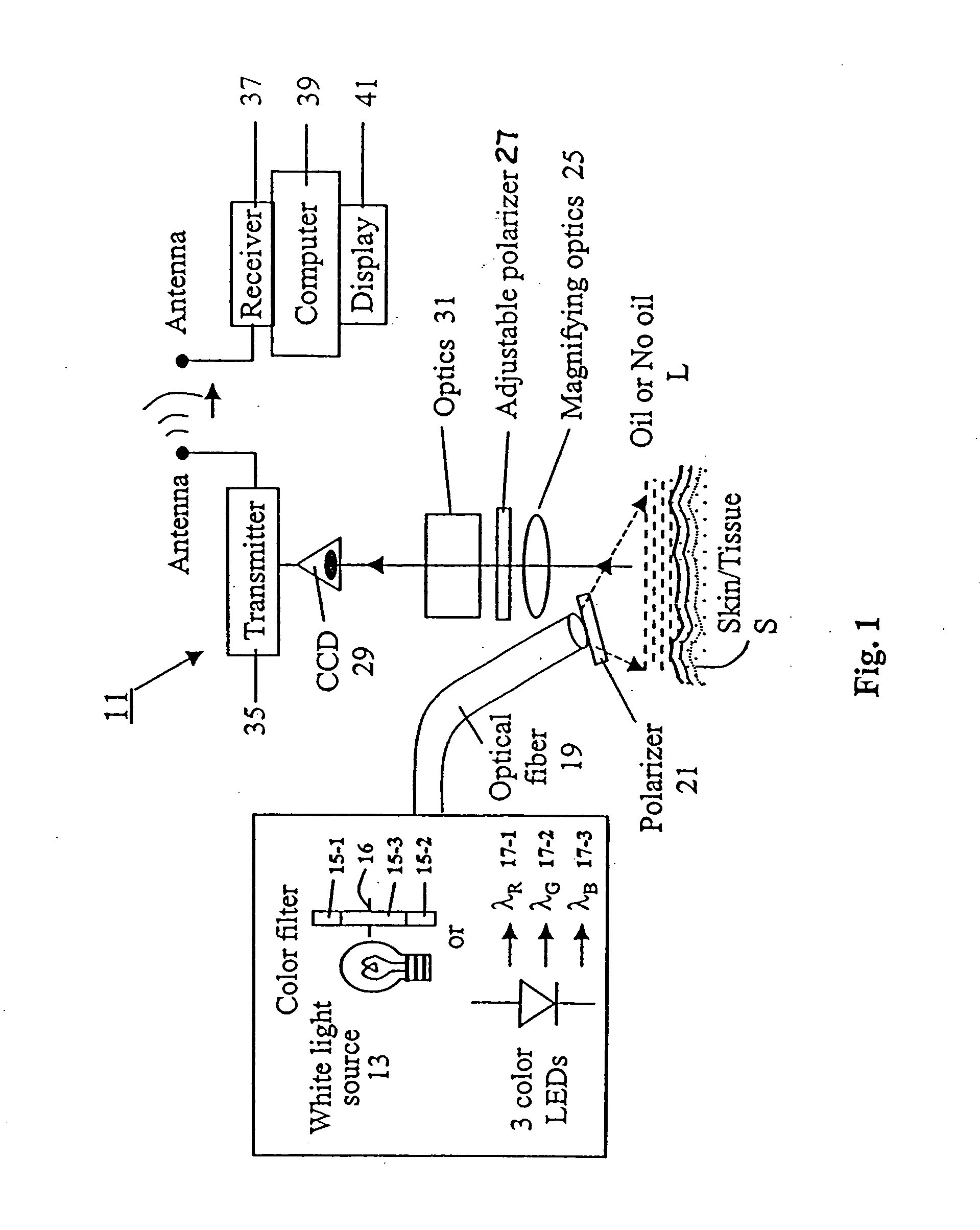
Spectral polarizing tomographic dermatoscope
An apparatus for use in examining an object, such as skin, mucosa and cervical tissues for detecting cancer and precancerous conditions therein. In one embodiment, the apparatus includes a gun-shaped housing having a handle portion and a barrel portion. The front end of the barrel portion is open, and a glass cover is mounted therein. LED's are disposed within the handle portion. A manually-operable switch for controlling actuation of the LED's is accessible on the handle portion. An optical fiber is used to transmit light from the LED's through a first polarizer in the barrel portion and then through the glass cover to illuminate a desired object. Reflected light from the object is passed through a second polarizer, which is adjustably mounted in the barrel portion and which is preferably oriented to pass depolarized light emitted from an illuminated object, and is then imaged by optics onto a CCD detector. The detector is coupled to a wireless transmitter that transmits the output from the detector to a remotely located wireless receiver.
Owner:ALFANO ROBERT R +3
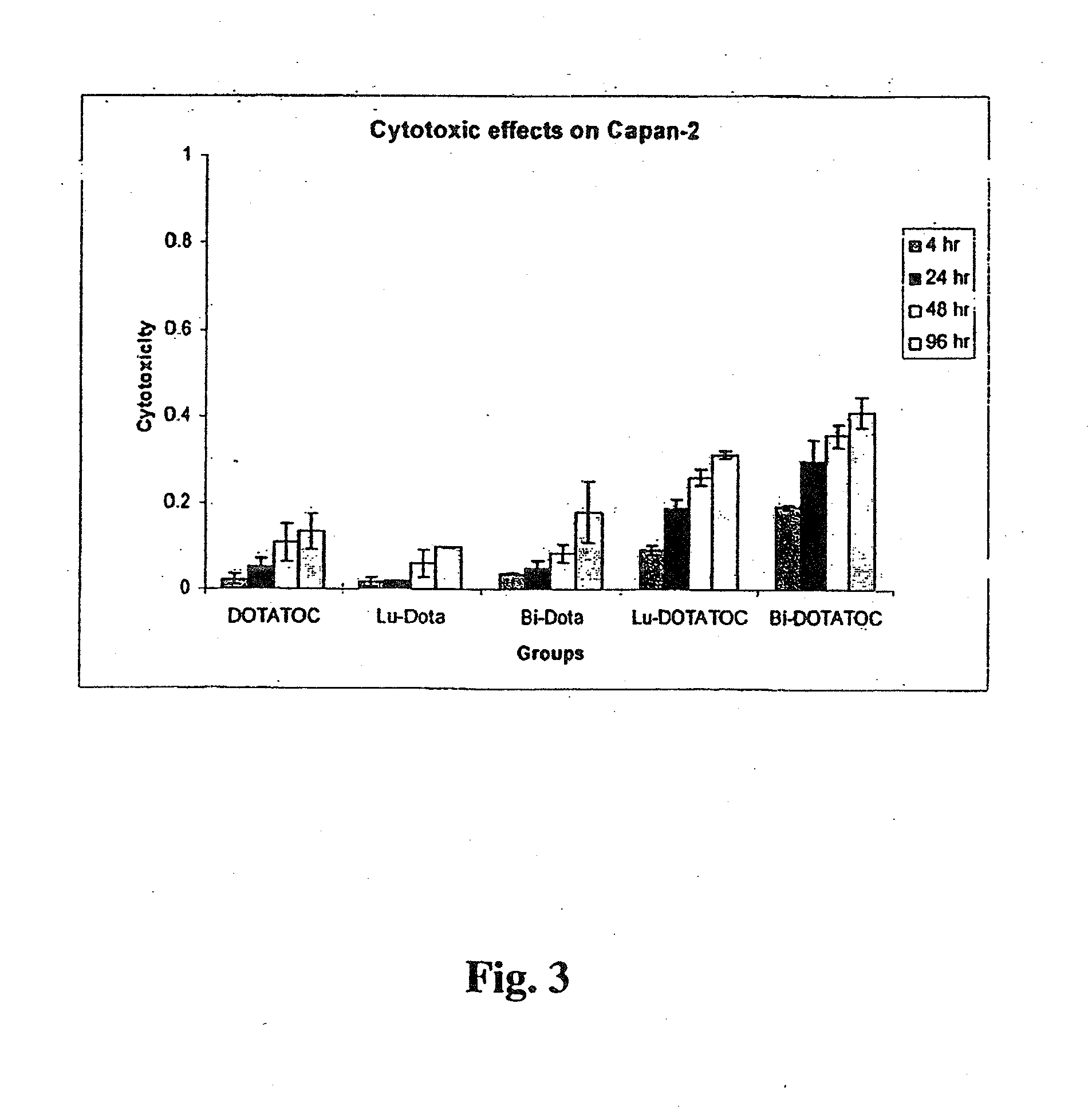
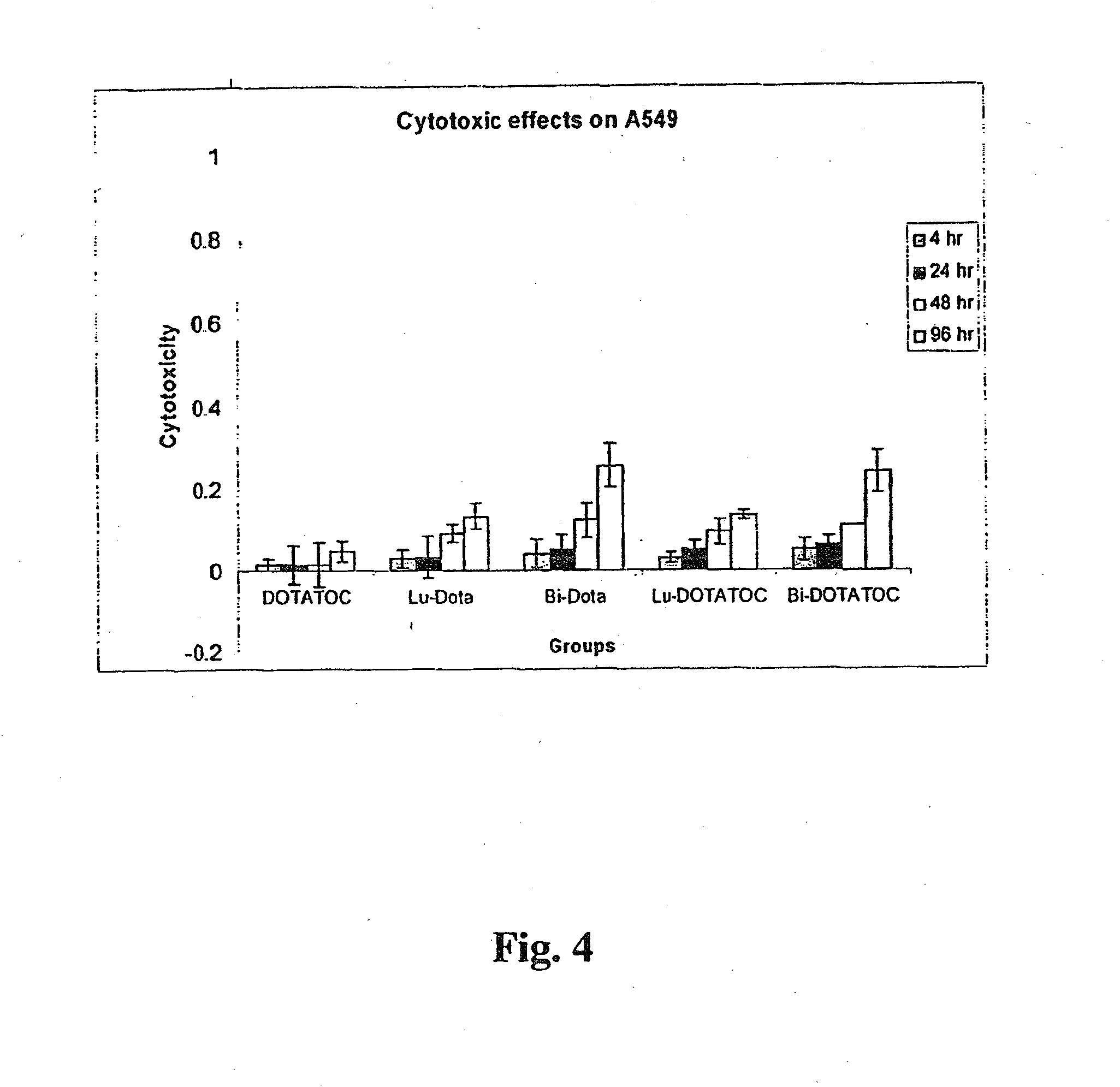
Anticancer therapy
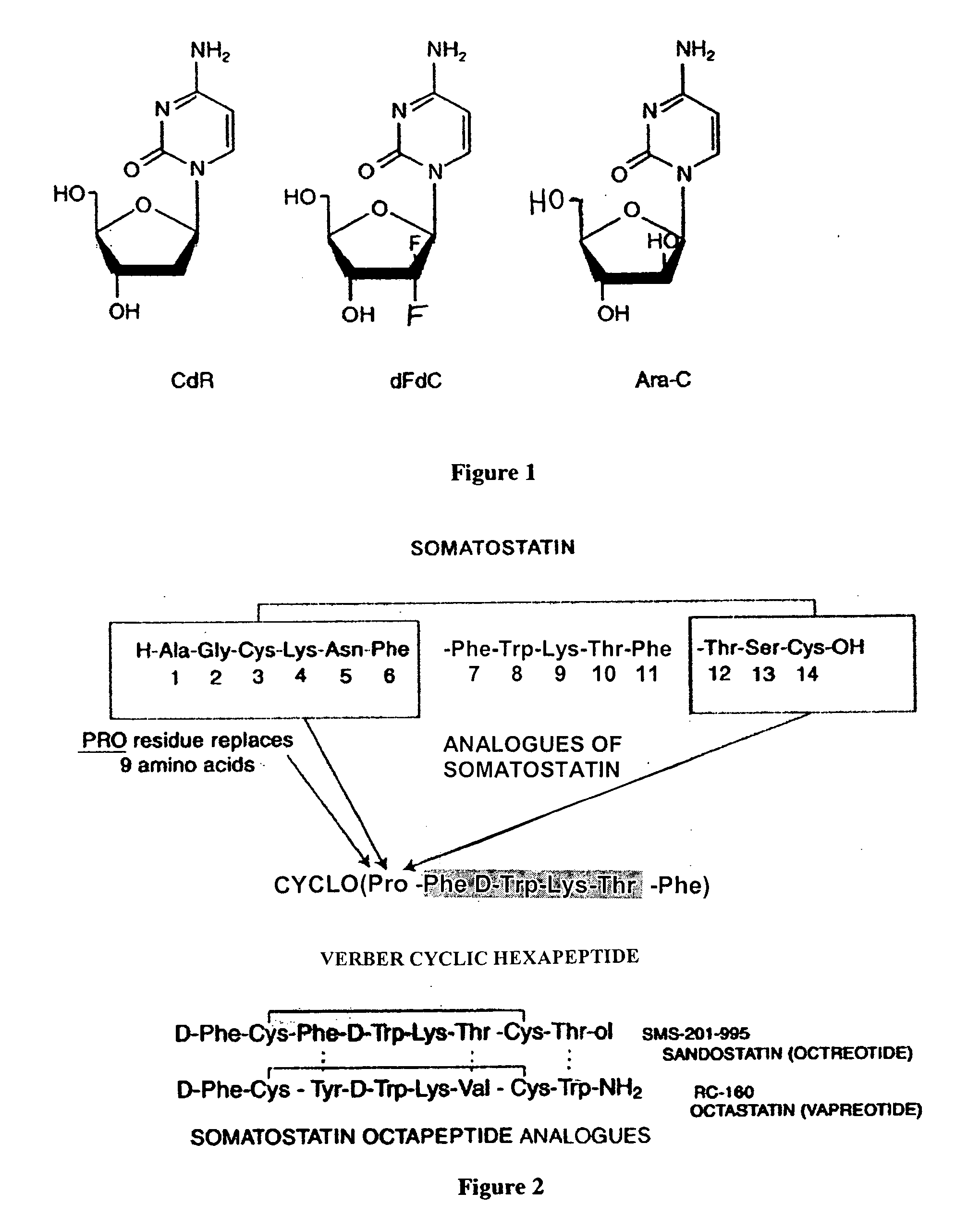
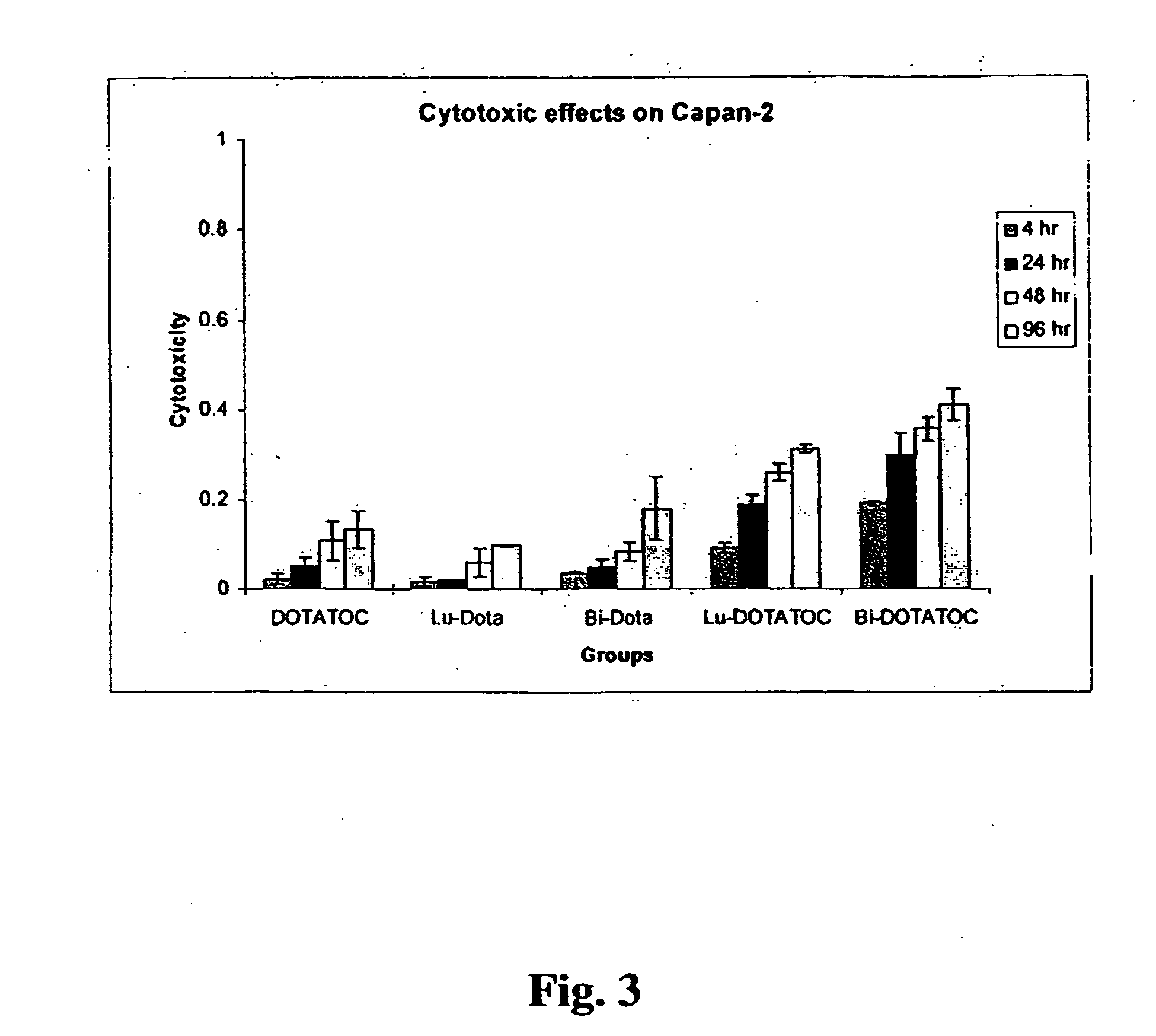
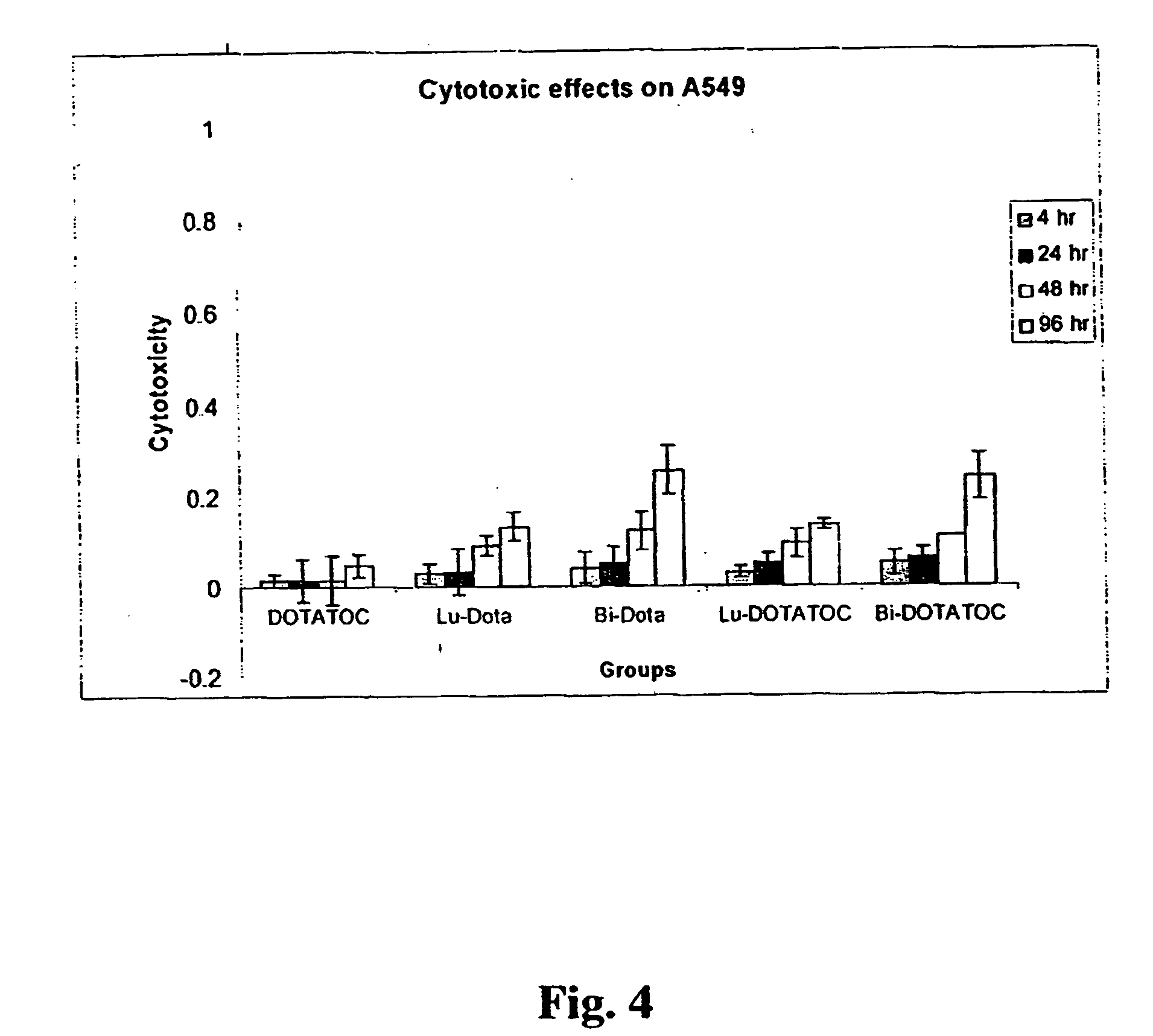
InactiveUS20070025910A1High expressionReduce the possibilityOrganic active ingredientsPeptide/protein ingredientsSomatostatin analogCytotoxicity
A subject afflicted with a cancer or precancerous condition is treated by administering an agent that increases expression of somatostatin receptors, and a cytotoxic recognition ligand. In an alternative embodiment, somatostatin analogs, which are radiolabeled are used to treat cancer or precancerous conditions.
Owner:STC UNM
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents, especially for cervical cancers and dysplasias
InactiveUS6462064B1Easy to convertCombating the greasy appearance of the skin orBiocideHydroxy compound active ingredientsCancer preventionRetinoid
The invention relates to the discovery that specific adamantyl or adamantyl group derivatives containing retinoid-related compounds induce apoptosis of cancer cells and therefore may be used for the treatment of cancer, including advanced cancer. Also, the present invention relates to novel adamantyl or adamantyl group derivatives containing retinoid compounds and their usage for treatment and / or prevention of cancer, keratinization disorders, dermatological conditions, and other therapies More specifically, it has been shown that such adamantyl compounds, e.g., 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid, 2-[3-(1-adamantyl)-4-methoxyphenyl]-5-benzimidazole carboxylic acid, and 6-[3-(1-adamantyl)-4,5-methylenedioxyphenyl]-2-naphthoic acid, can be used to treat or prevent cervical cancers and precancers such as cervical dysplasias, including high grade and low grade dysplasias.
Owner:GALDERMA RES & DEV SNC +1
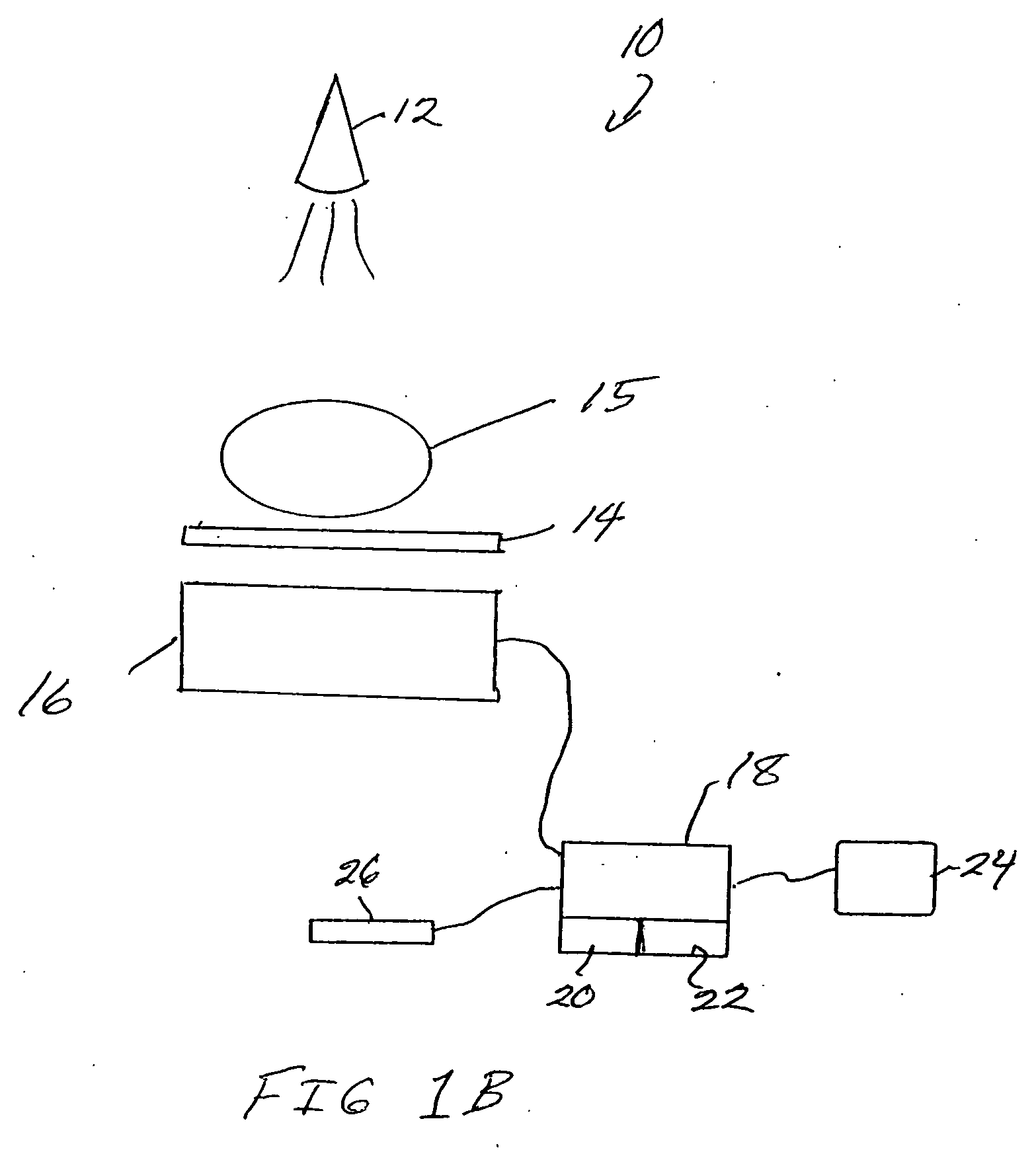
Oral cancer screening device
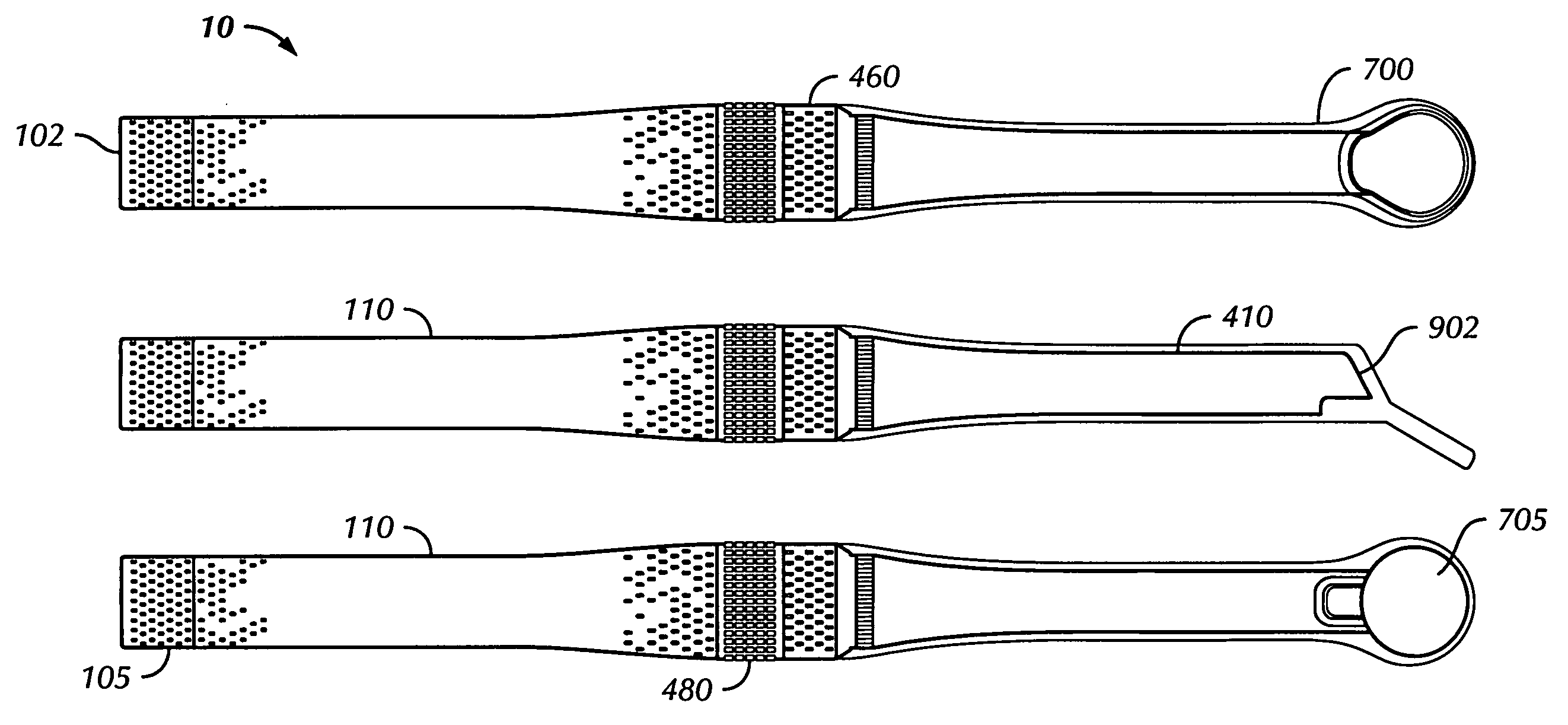
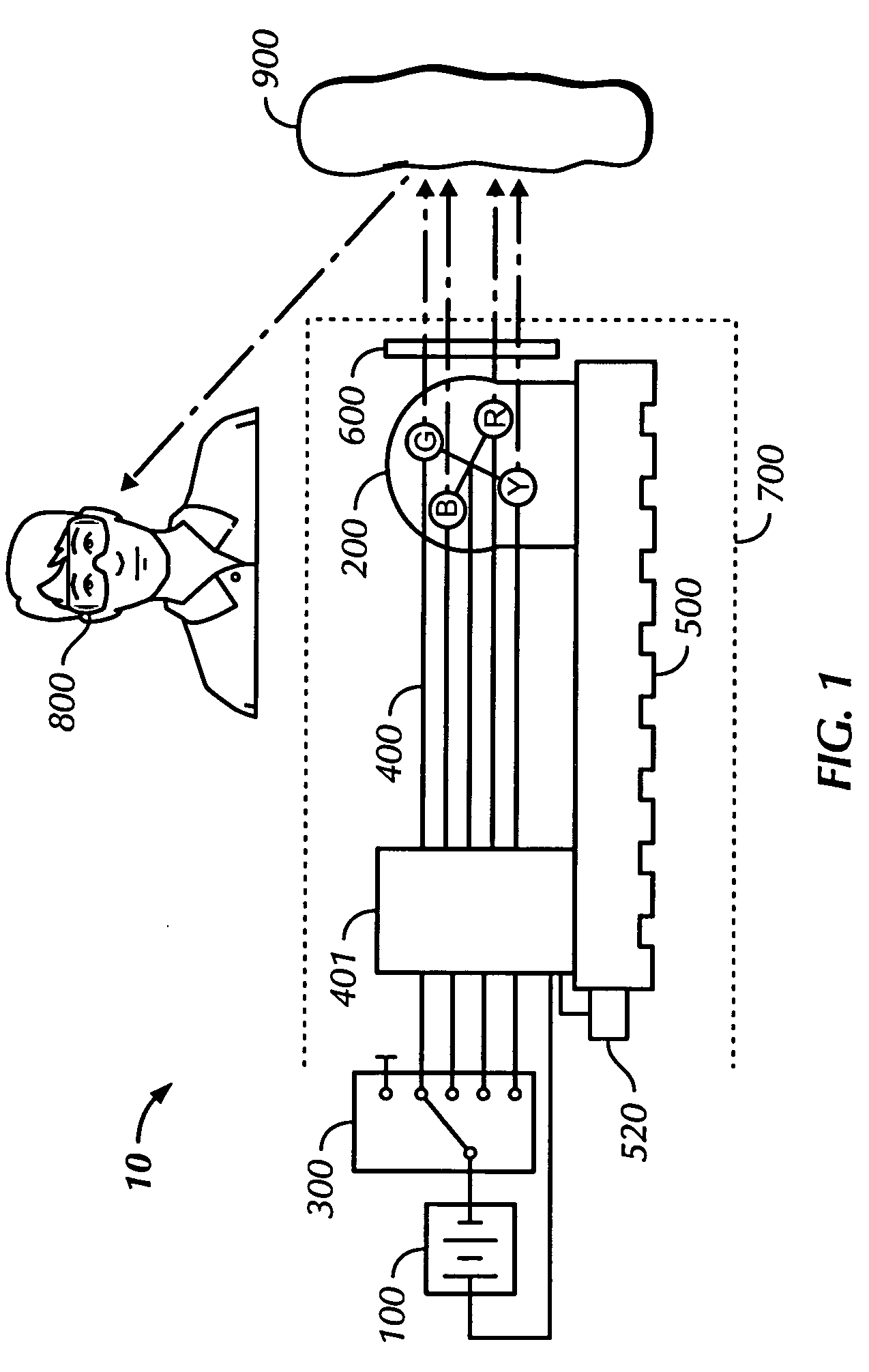
An oral cancer screening device 10 used for the detection of pre-cancerous and cancerous tissue has a power supply 100, an illumination source 200, a selector switch 300 that enables the activation of a specific wavelength of light from the illumination source 200, an electrical system 400 in communication with the selector switch 300 and the illumination source 200, a heat sink 500 in thermal communication with the illumination source, a filter or cover 600 to protect the illumination source, and a transparent sheath 700 for providing a sanitary shield for the device when it is brought into contact or close proximity with the patient oral cavity. The sheath may optionally have an angled mirror incorporated at a distal end of the sheath to provide the operator with a reflected image of the illuminated tissue 900. The operator will optionally utilize head mounted lenses 800 to assist the operator's visualization of the light from the illuminated oral cavity.
Owner:REMICALM LLC
Biomarkers for Head-And-Neck Cancers and Precancers
The invention provides markers and methods for detecting head-and-neck precancers, (including OPLs), cancers and related disease conditions in a subject. The invention also provides localization and imaging methods for head-and-neck precancers (including OPLs) and cancers, along with kits for carrying out methods of the invention. The invention further provides therapeutic applications for head-and-neck precancers (including OPLs) and cancers which employ head-and-neck precancer and cancer markers, polynucleotides encoding the markers, and binding agents for the markers.
Owner:WALFISH PAUL +1
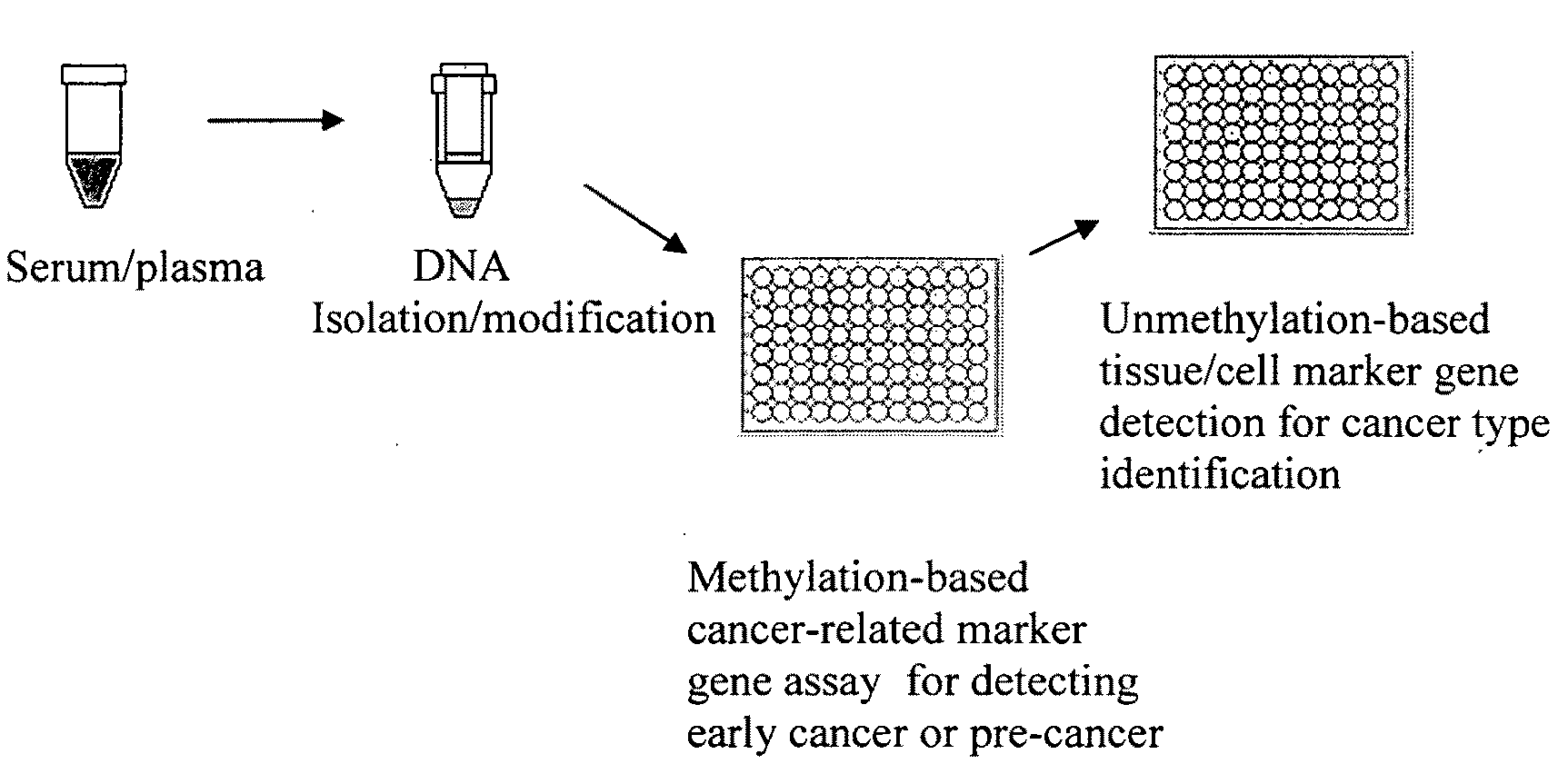
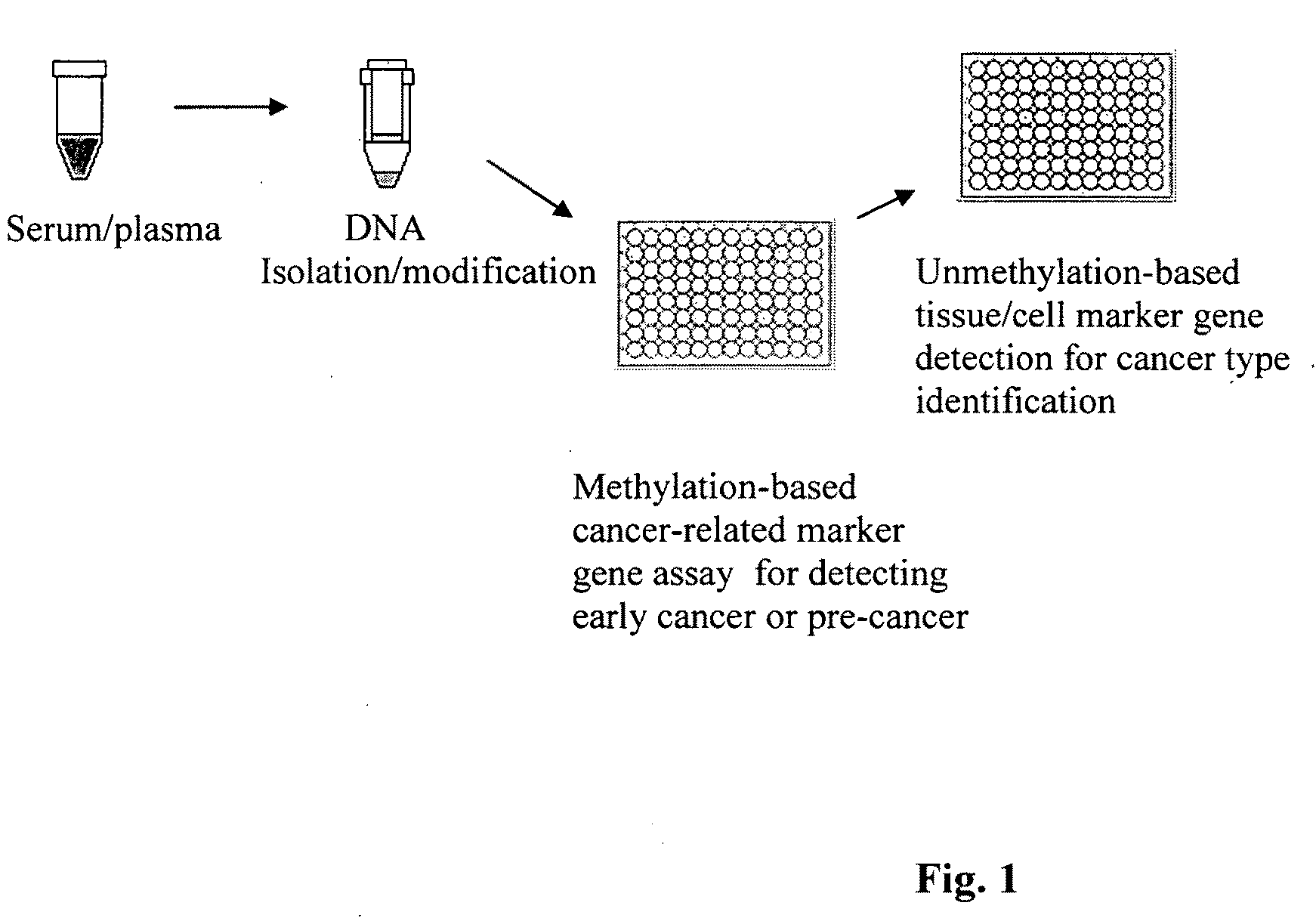
Method and kit for detection of early cancer or pre-cancer using blood and body fluids
Owner:LI WEIWEI +1
Anticancer therapy
ActiveUS20150196673A1High expressionReduce the possibilityOrganic active ingredientsPeptide/protein ingredientsSomatostatin AnalogueSomatostatin receptor
A subject afflicted with a cancer or precancerous condition is treated by administering an agent that increases expression of somatostatin receptors, and a cytotoxic recognition ligand. In an alternative embodiment, somatostatin analogs, which are radiolabeled are used to treat cancer or precancerous conditions.
Owner:STC UNM
Tissue chip used for tumour early stage diagnosis and preparation device
Three kinds of tissues including cancer tissue, precancerosis and corresponding normal tissue are sliced up, dyed, marked, and positioned. Receptor holes are prepared by leading designed lattice array mould paper to paste on surface of wax block of receptor. Wax block with tissue core bar is prepared by using perforating needle and puncture needle for tissue. Common cancer such as lung cancer, nasopharyngeal carcinoma, oesophagus cancer etc. and having integrated clinical data and pathology features are selected. Through in situ hybridization, testing mRNA of relevant gene and expression of protein on tissue chip, consistent result between the invented product and traditional test is validated. In the product, cellular morphology is clear and even, and there is no fallen off tissue point. The invention is applicable to filter cancers, early diagnosis and forecasting prognosis.
Owner:中南大学湘雅医学院肿瘤研究所
Stromal collagen in the diagnosis and characterization of breast cancer
ActiveUS7805183B2Test accurateGood informationImage enhancementRadiation pyrometryAbnormal tissue growthPresent method
The present invention provides methods and systems for evaluating biological materials for the diagnosis of disease, such as gland abnormalities, and the cancerous and precancerous conditions. Nonlinear optical microscopy techniques, such as MP microscopy and harmonic generation microscopy, are used to generate high resolution, three dimensional images of a test tissue, such as a biopsy tissue sample and tissue in whole organisms, that are analyzed, optionally in combination, to detect, identify and characterize tumor-associated collagen signatures. The presence, abundance and extent of histological features and structural motifs comprising tumor-associated collagen signatures may be directly and accurately correlated with the onset and progression of cancer, such as breast cancer. The present methods are capable of providing an accurate and selective diagnosis of cancer, and provide diagnostic information complementary to conventional diagnostic methods.
Owner:WISCONSIN ALUMNI RES FOUND
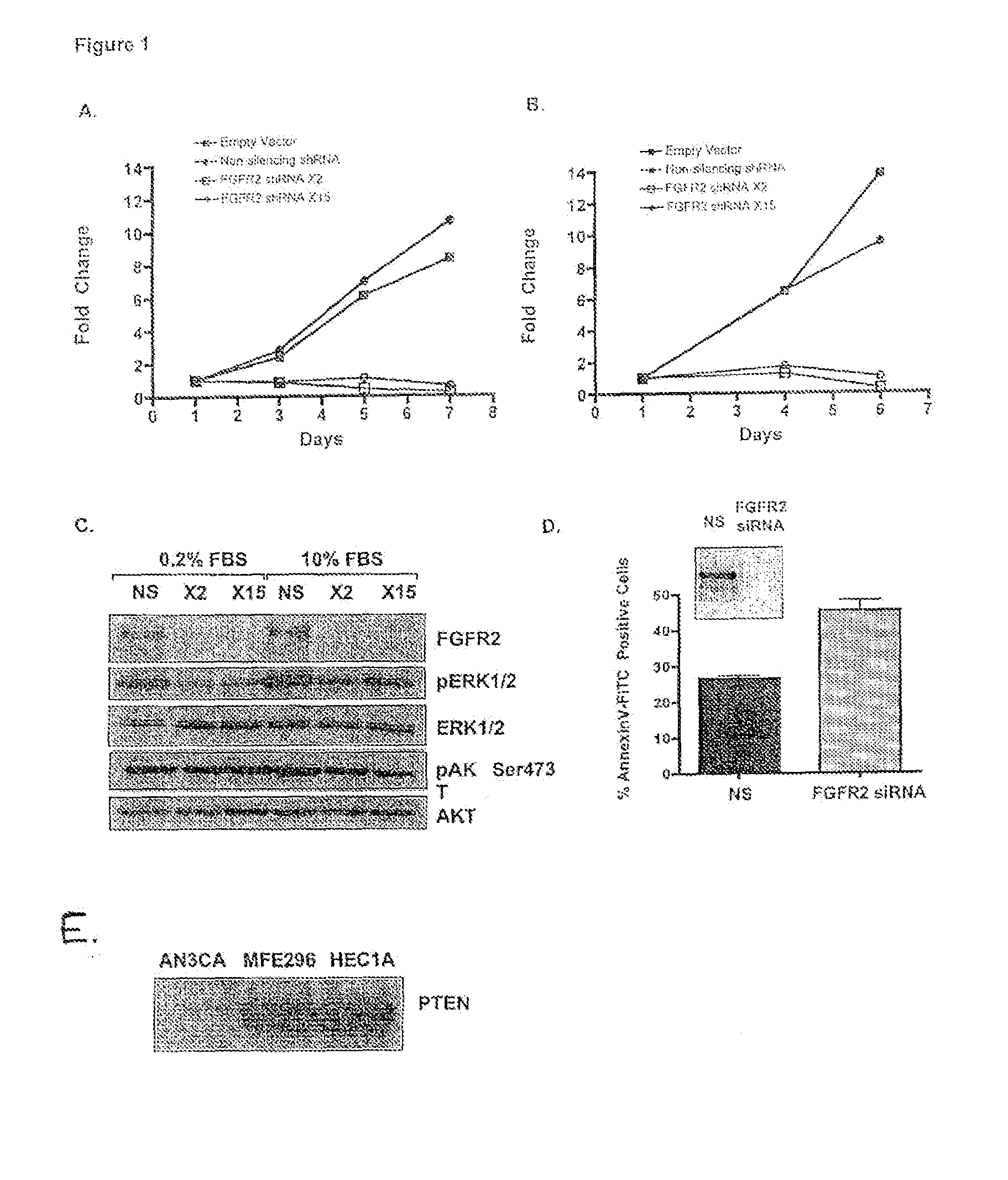
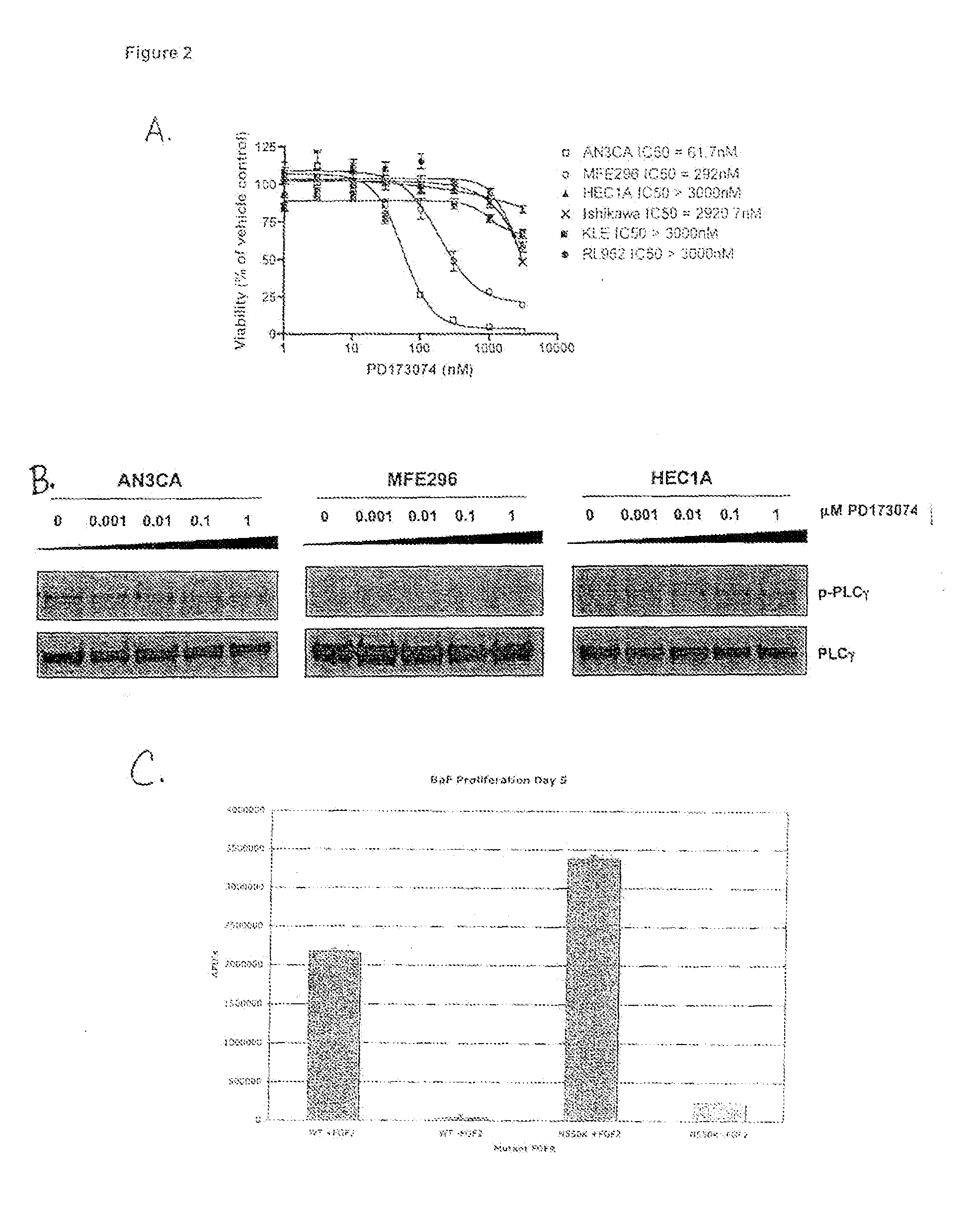
Method of diagnosing, classifying and treating endometrial cancer and precancer
InactiveUS20100111944A1Accurate classificationEnhanced ligand bindingOrganic active ingredientsSugar derivativesBiological activationNucleotide sequencing
Diagnostic and therapeutic applications for endometrial cancer are described. The diagnostic and therapeutic applications are based on certain activation mutations in the FGFR2 gene and its expression products. The present invention is directed to nucleotide sequences, amino acid sequences, probes, and primers related to FGFR2 activation mutants and kits comprising these mutants to diagnosis and classify endometrial cancer in a subject.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Method for building spleen-deficiency chronic atrophic gastritis gastric precancerous lesions animal model
InactiveCN102648701ASyndrome of spleen deficiencyHave body temperatureIn-vivo testing preparationsAnimal husbandryNitrosoRat model
The invention relates to a method for building a spleen-deficiency chronic atrophic gastritis gastric precancerous lesions animal model. The method is based on the existing spleen-deficiency rat model and the existing chronic atrophic gastritis rat model and is used for building the animal model through united copy of free drinking of N-methyl-N '-nitro-N -nitrosoguanidine (MNNG) solution, irregular eating and purgation. The method comprises the following specific steps of: preparing 10mg / L MNNG storage solution by utilizing sterilized tap water, preparing 80 to 200 mug / mL MNNG solution in temporary use to substitute the drinking water to be drunk freely by the rats, and adopting the free drinking of MNNG as the beginning of the modeling; copying a spleen-deficiency syndrome through irregular eating and improved purgation way, feeding the rats on alternate days since the fifth week after the modeling, and utilizing qi-activating decoction for gavage in a dose of 1 to 3ml per rat every day in the seventh weak; and continuing the modeling for 12 weeks. The animal model built through the method is identical to or similar with the clinical syndrome and physical signs of middle-late lesions in chronic atrophic gastritis. After being verified in repeatedly modeling, the method has good repeatability and good stability. The built model is suitable for selecting traditional Chinese medicine for treating the chronic atrophic gastritis through a traditional Chinese medicine spleen treatment method.
Owner:潘华峰
Compositions and methods for detecting pre-cancerous conditions in cell and tissue samples using 5, 10, 15, 20-tetrakis (carboxyphenyl) porphine
InactiveUS6838248B2Organic active ingredientsPreparing sample for investigationState dependentFluorescence
Presented is a method to detect precancerous states in mammalian cell and tissue samples comprising incubating a sample with solubilized 5, 10, 15, 20-tetrakis (carboxyphenyl) porphine (TCPP), measuring TCPP fluorescence in the sample, and categorizing the sample as non-cancerous, precancerous or cancerous based on TCPP fluorescence, as correlated with the respective states of the cells. In conjunction with the method a detection, a novel and more efficient method of solubilizing TCPP is presented, as well as a composition comprising TCPP solubilized by this method.
Owner:BIOAFFINITY TECH
Methods for detection of breast cancer
InactiveUS20060246415A1Facilitate early detectionReduce in quantityMicrobiological testing/measurementBiological testingOncologyDisease status
The invention relates to a simple screening test for neoplasia, a precancerous condition, or cancer of the breast. A method is described whereby a breast cancer marker is detected in breast fluid. In a particular embodiment, the method involves treating samples of breast fluids with an aldehyde detecting reagent without any prewashing. The appearance in breast fluids of a marker that is detected by an aldehyde detecting reagent, such as a Schiff s reagent, correlates very well with the disease status of the breast cancer subjects from which the fluids were obtained. Screening test kits are also provided.
Owner:SENTINA BIOTECH
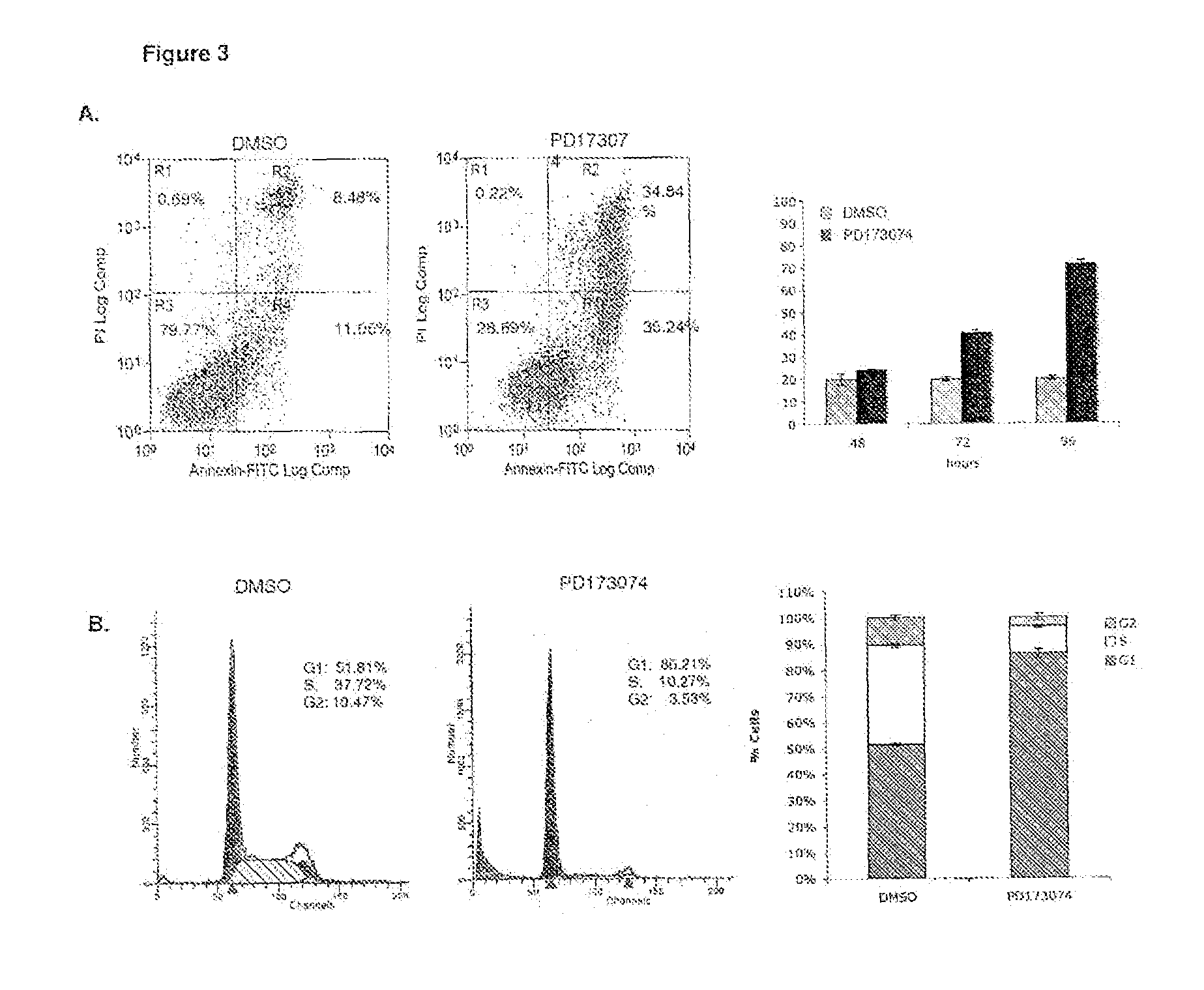
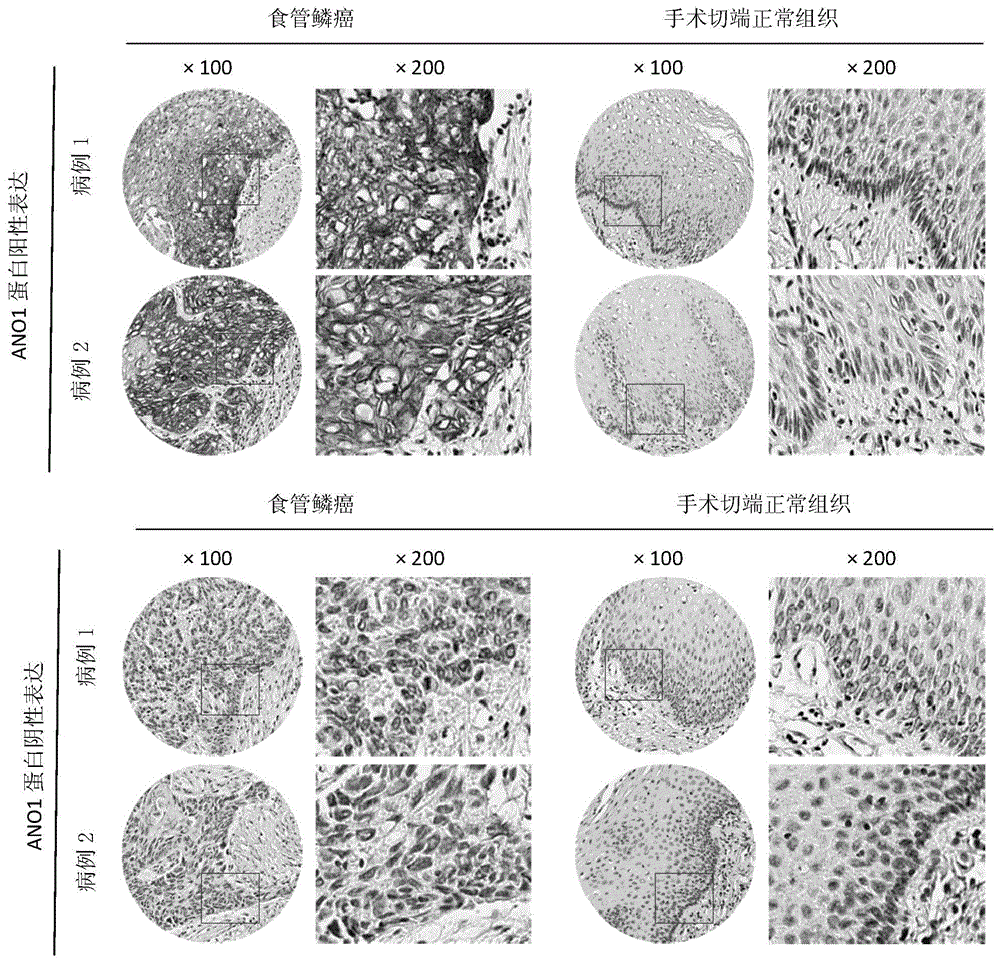
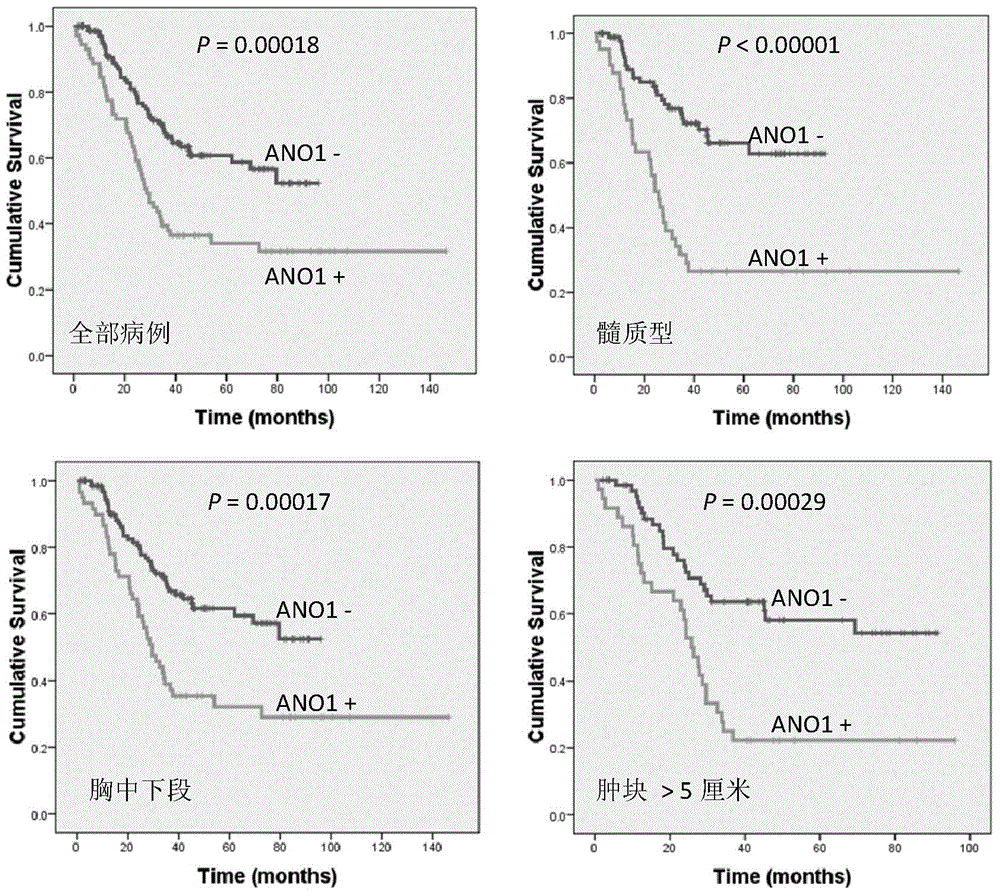
Application of ANO1 protein in prediction on prognosis of esophagus cancer and precancerous lesion risk
The invention belongs to the field of prognosis estimation and canceration risk prediction on clinic of molecular biology, and particularly relates to application of a molecule of a detection ANO1 protein in the preparation of a kit or a detection agent for the prognosis estimation and precancerous disease progression risk prediction of esophageal squamous carcinoma. The molecule expressed by the detection ANO1 protein can be a nucleic acid, a protein or a compound, thus showing that the ANO1 protein can be used as a molecular marker for the prognosis estimation and precancerous disease progression risk prediction of the esophageal squamous carcinoma.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Compositions, kits, and methods for identification, assessment, prevention, and therapy of cervical cancer
InactiveUS20080286781A1Reduced expression levelOrganic active ingredientsGenetic material ingredientsCervical caOncology
Owner:MILLENNIUM PHARMA INC
Organoselenium compounds for cancer chemoprevention
InactiveUS7087639B2Enhance chemopreventive effect of compoundLower metabolismBiocidePeptide/protein ingredientsFood additiveChemical compound
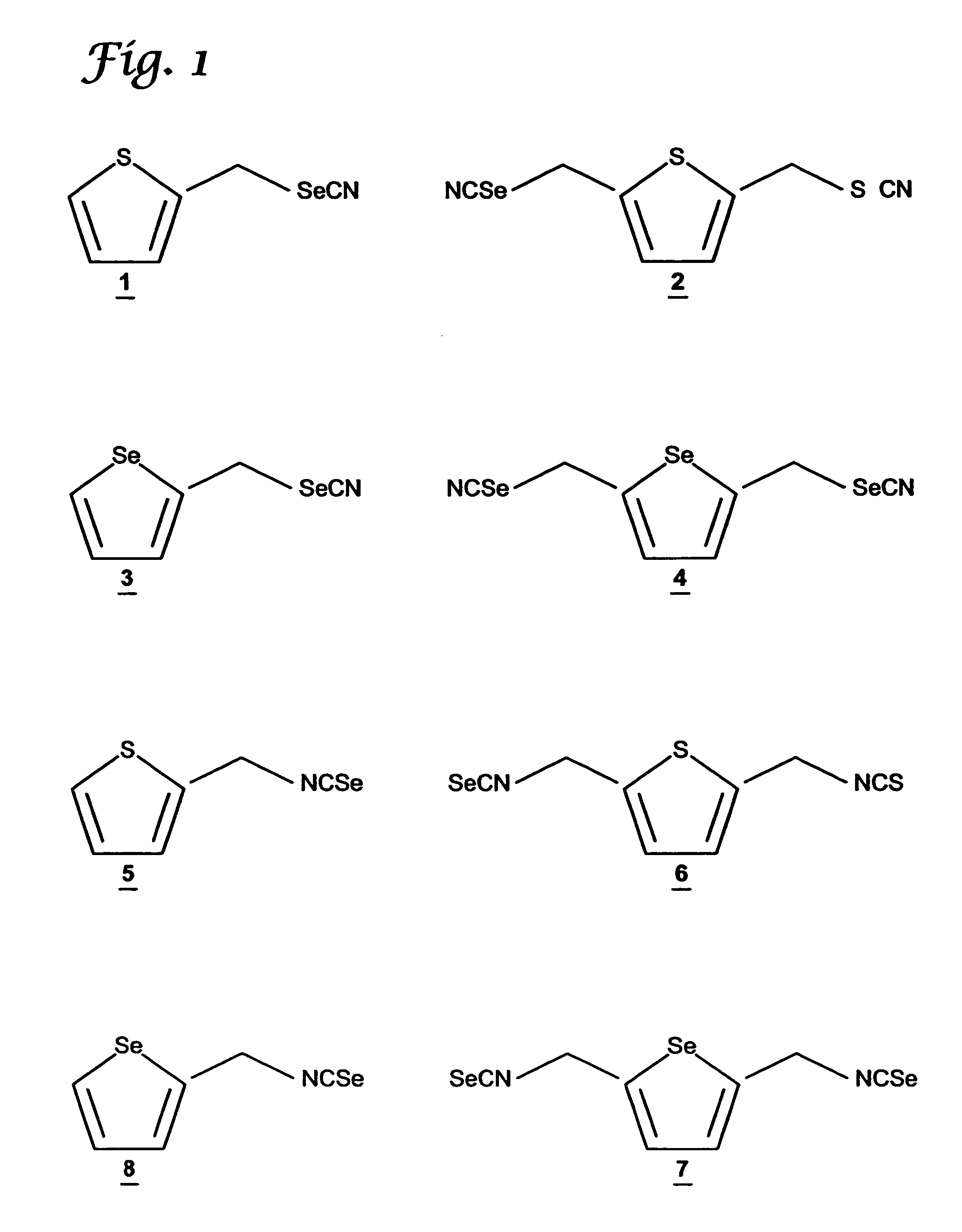
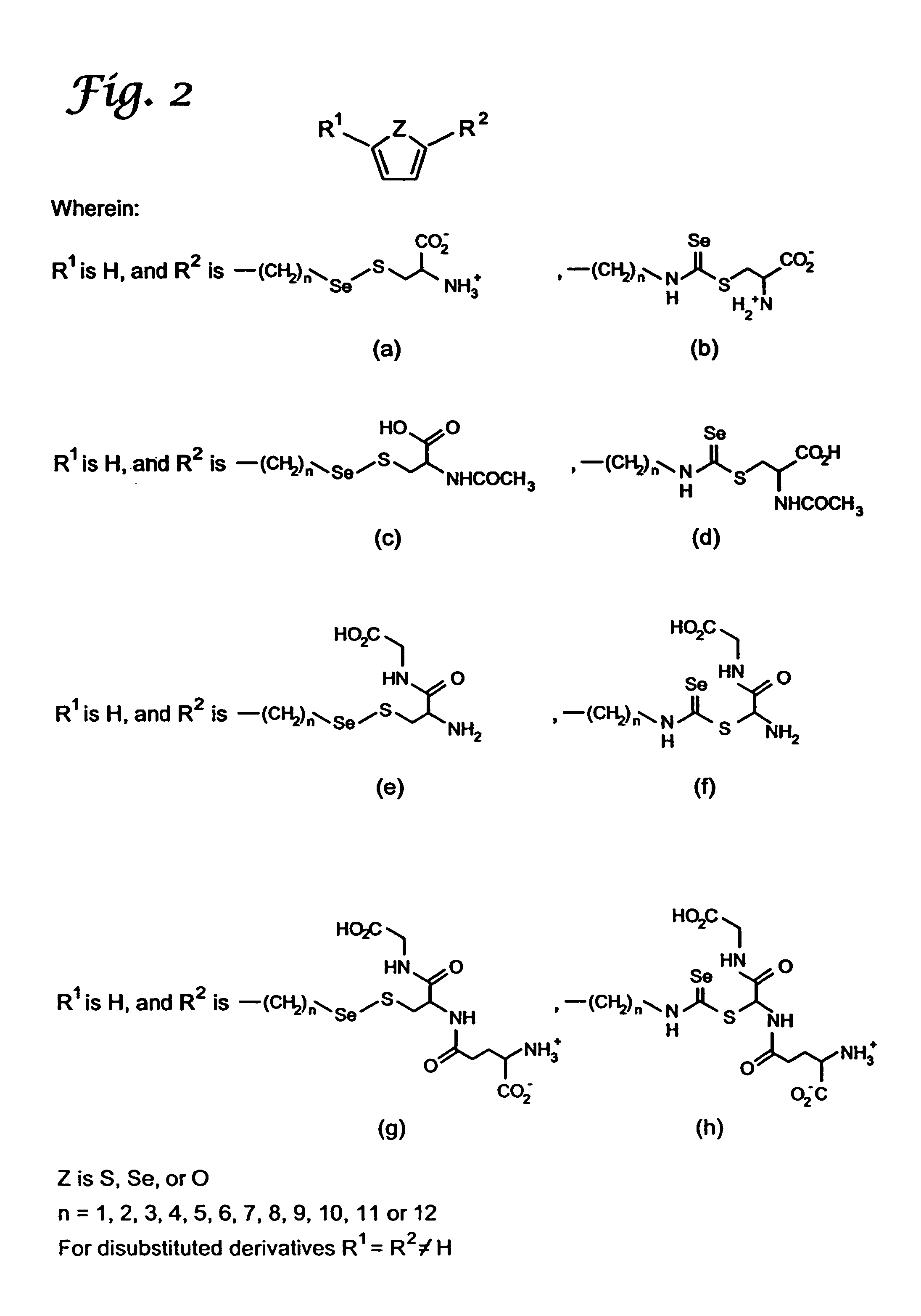
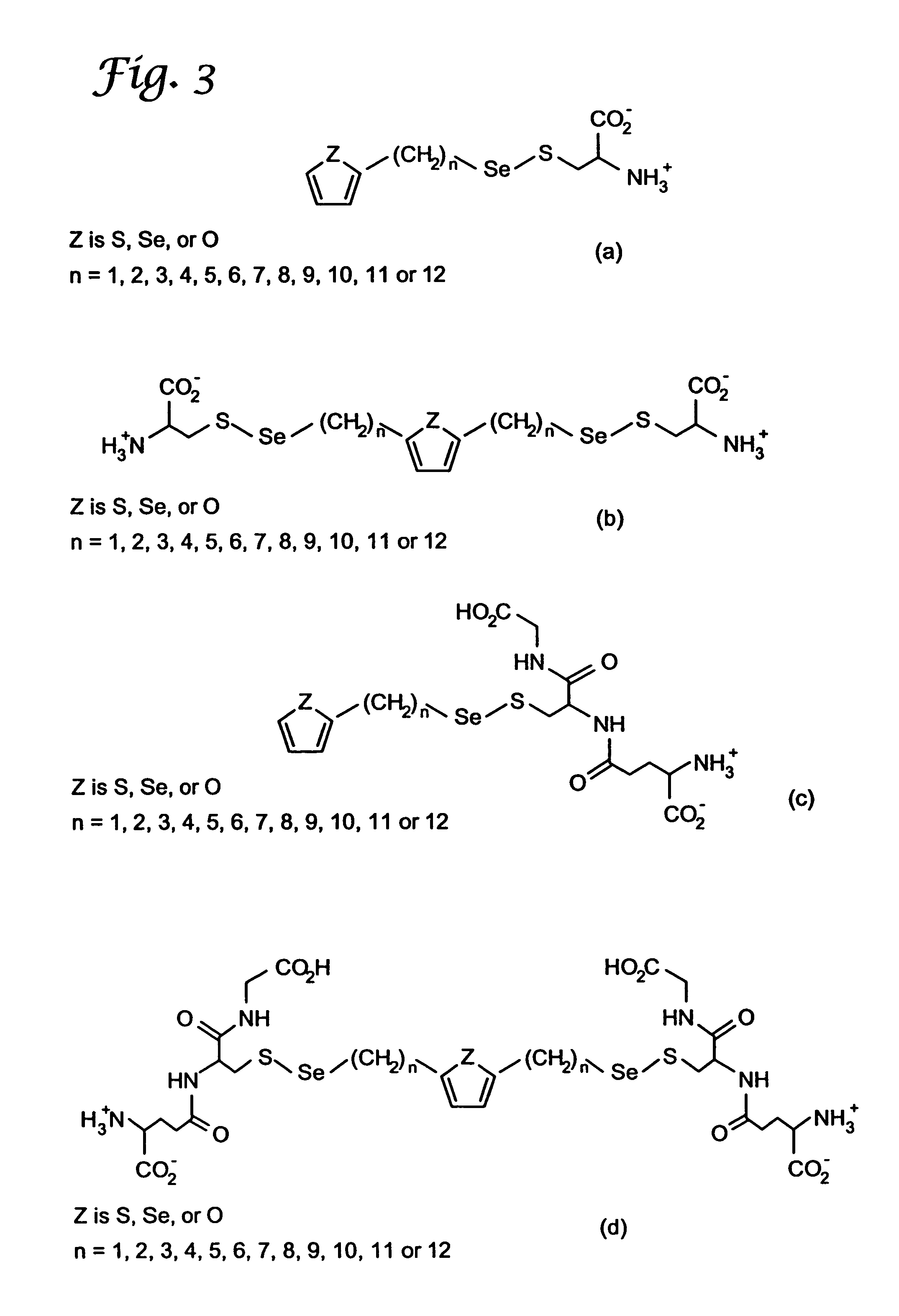
A compound containing an alkylene selenocyanate or an alkylene isoselenocyanate moiety effective to prevent the occurrence or progression of cancer or a precancerous condition. The compound can be provided and administered in the form of a pharmaceutical composition, a cosmetic, a food additive, supplement, or the like. Methods for synthesis and use of the chemopreventive compound of the invention are also provided.
Owner:LKT LAB
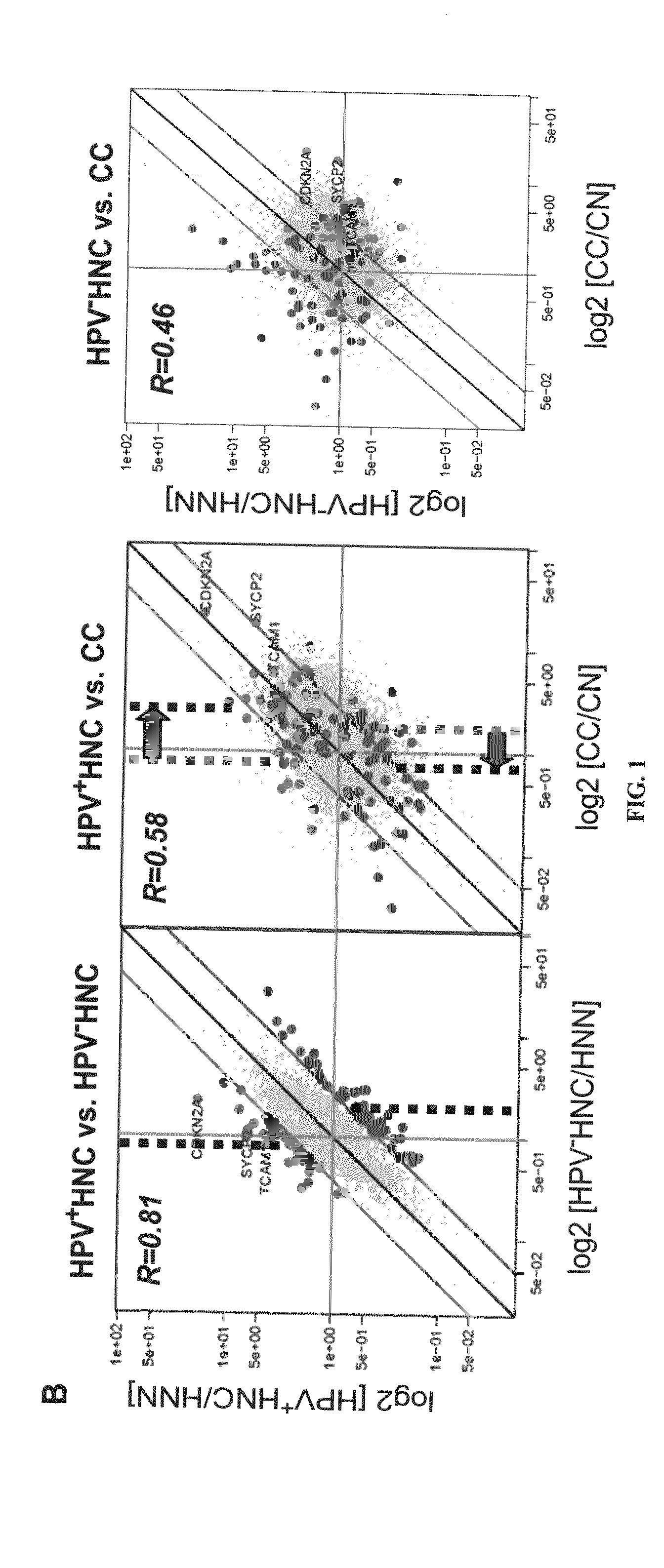
Biomarkers for human papilloma virus-associated cancer
ActiveUS20090136486A1Microbiological testing/measurementAntibody ingredientsSynaptonemal complex protein 2Antigen
Cervical cancer cells and HPV+ head and neck cancer cells express three testis-specific genes not normally expressed in somatic cells: testicular cell adhesion molecule 1 (TCAM1), synaptonemal complex protein 2 (SYCP2) and stromal antigen 3 (STAG3). Among the three markers, TCAM1 and SYCP2 are early detection markers. Various methods for identifying a human or non-human animal as a candidate for further examination for cervical cancer, preneoplastic lesion for cervical cancer, head and neck cancer, or preneoplastic lesion for head and neck cancer are disclosed. Methods of detecting said cancers and preneoplastic lesions, methods of screening for drugs for treating said cancers and preneoplastic lesions, methods for monitoring the effectiveness of a treatment for said cancers, and methods of treating said cancers are also disclosed. Further disclosed are kits that can be used to practice the above methods.
Owner:WISCONSIN ALUMNI RES FOUND
Compositions and methods for oral cancer chemoprevention using berry preparations and extracts
Owner:UNIV OF KENTUCKY RES FOUND +1
Compounds and compositions for use in the prevention and treatment of inflammation-related disorders, pain and fever, skin disorders, cancer and precancerous conditions thereof
InactiveUS20140315834A1Improve efficacyImprove securityBiocideSugar derivativesThioester synthesisSide effect
The present invention provides novel compounds and pharmaceutical compositions for the prevention and / or treatment of cancer and precancerous conditions thereof, for the treatment of pain and fever, for the treatment of skin disorders, and for treating and / or preventing inflammation-related diseases and / or cardiovascular diseases. The compounds of the invention also have analgesic properties and anti-platelet properties. The compounds of the invention may be provided to animals, including mammals and humans, by administering a suitable pharmaceutical dose in a suitable pharmaceutical dosage form. The compounds of the invention have improved efficacy and safety, including higher potency and / or fewer or less severe side effects, than conventional therapies. The compounds of the invention comprise a biologically active moiety or portion (A) that has, or is modified to have at least one carboxyl group. The moiety A is preferably an aliphatic, aromatic or alkylaryl group, preferably derived from a non-steroidal anti-inflammatory drug or NSAID (A). The moiety A is bound to a linker moiety (B) via the carboxyl of group A and a linking atom that is selected from oxygen, nitrogen, and sulphur, to form a carboxylic ester, and amide, or a thioester, bond (X1) between groups A and B. Moiety B is a single bond, an aliphatic group, a substituted benzene, or an alkylene substituted hydrocarbon chain, which in turn is bound to functional moiety Z, which facilitates access of the compound into cells. The moiety Z can comprise, for example, a phosphorous-containing group, a nitrogen-containing group, or a folic acid residue.
Owner:MEDICON PHARMA
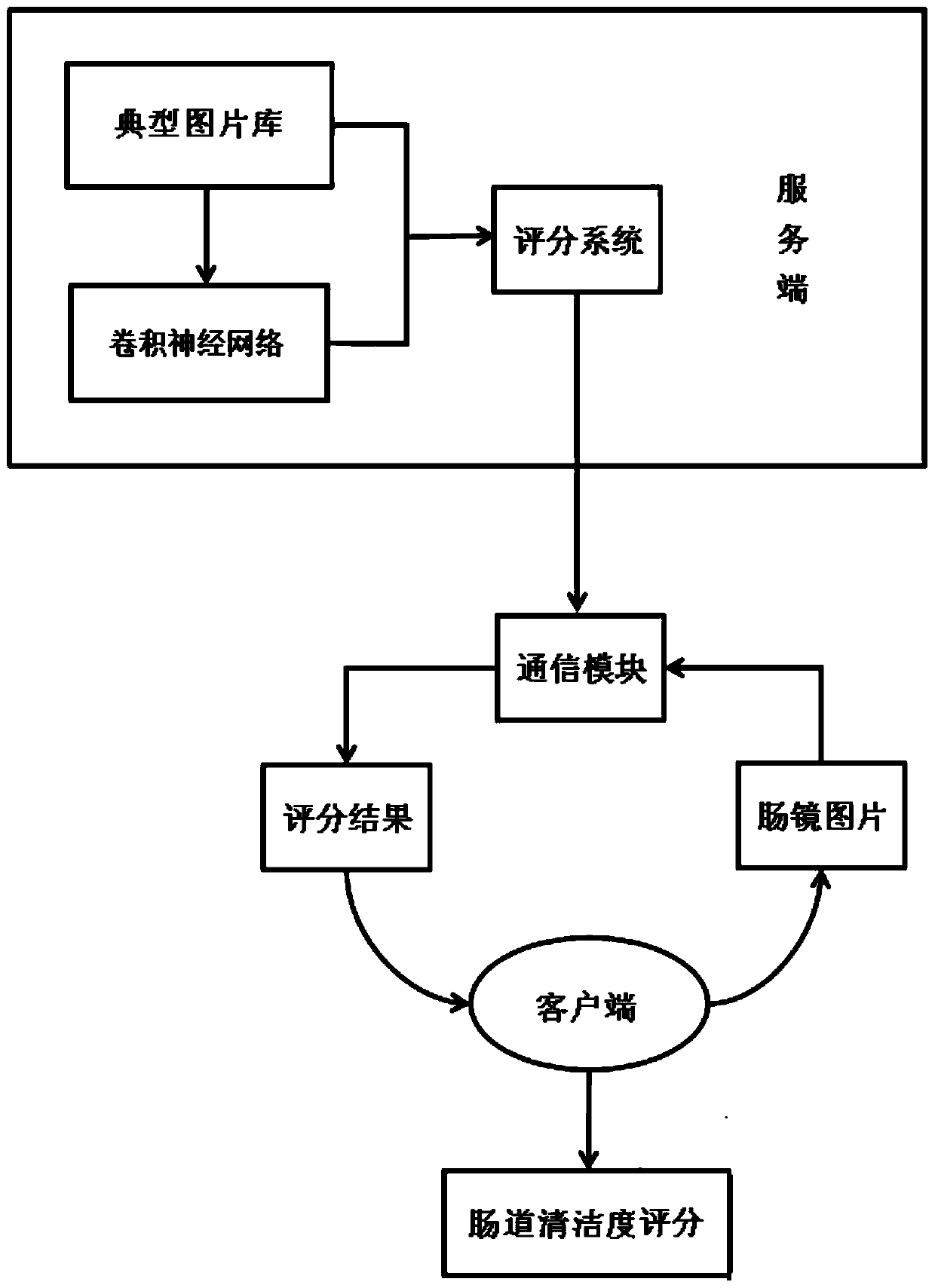
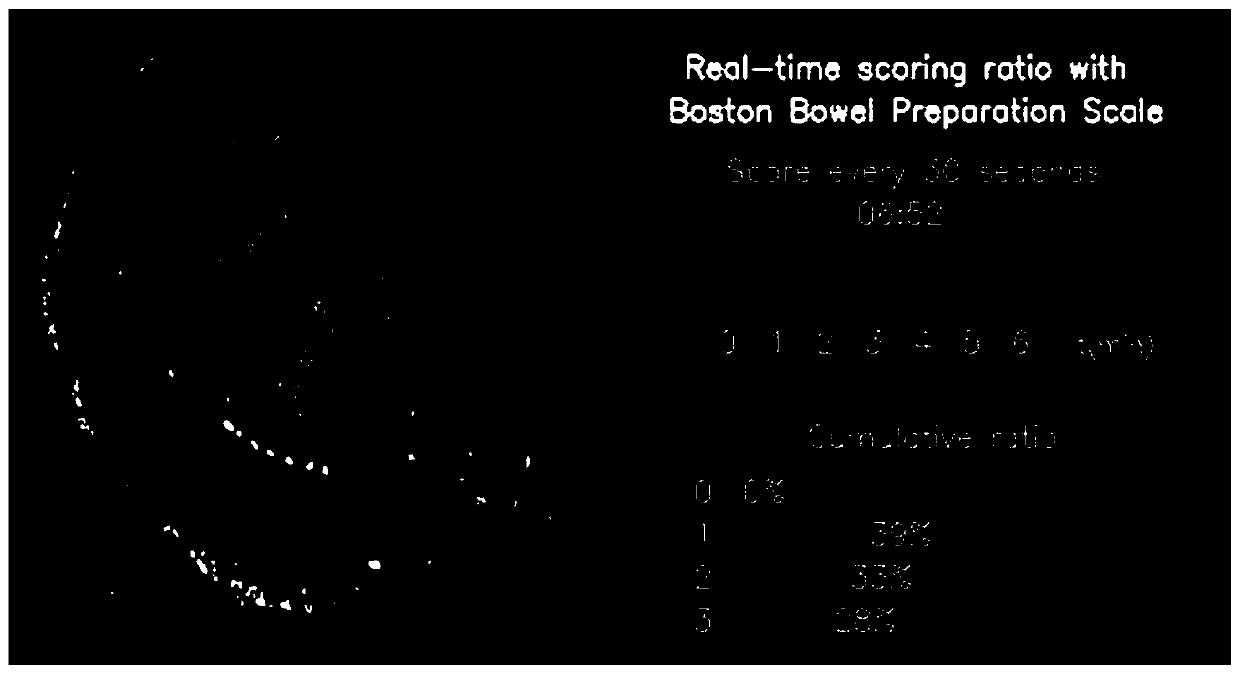
Real-time intestinal tract cleanliness scoring system and method based on artificial intelligence
InactiveCN110916606APrepare for quality understandingImprove preparation qualityEndoscopesRectum colonoscopesBowel preparationPrecancerous condition
The invention discloses a real-time intestinal tract cleanliness scoring system and method based on artificial intelligence. By adopting the system and method, bowel preparation quality examined by aclinical colonoscope is monitored in real time, and scoring display is carried out at a client; and the constituent ratio of each score and the cleanliness of an examined intestinal segment within each 30 s are represented. On one hand, the bowel preparation quality condition of a patient operated by a physician can be more objectively and directly expressed in a quantitative manner, and the workload and scoring errors of endoscopic physicians are reduced, so that the error evaluation of endoscopic examination quality is reduced and the interval of reexamination of colonoscope examination isrecommended; on the other hand, medical institutions can more objectively and directly know about the bowel preparation quality, so that the quality control work can be done effectively, the intestinal tract cleaning quality is rapidly improved, the adenoma detection rate is reduced, and early detection and early treatment of precancerous lesions of intestinal tracts are realized; and more importantly, the development of the system facilitates scientific inquiry for subsequent influences on bowel preparation schemes by different intestinal tract preparations.
Owner:WUHAN ENDOANGEL MEDICAL TECH CO LTD
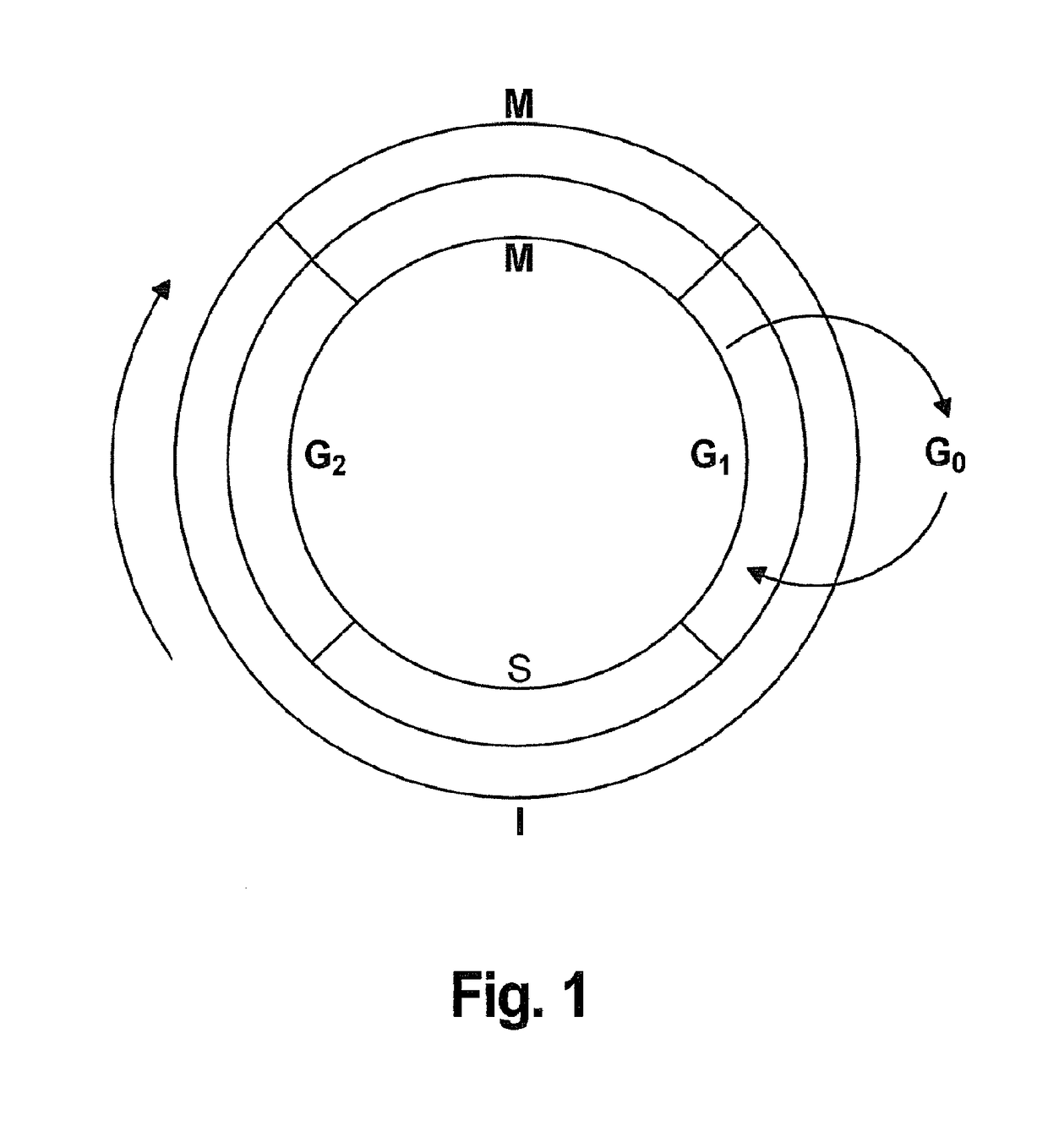
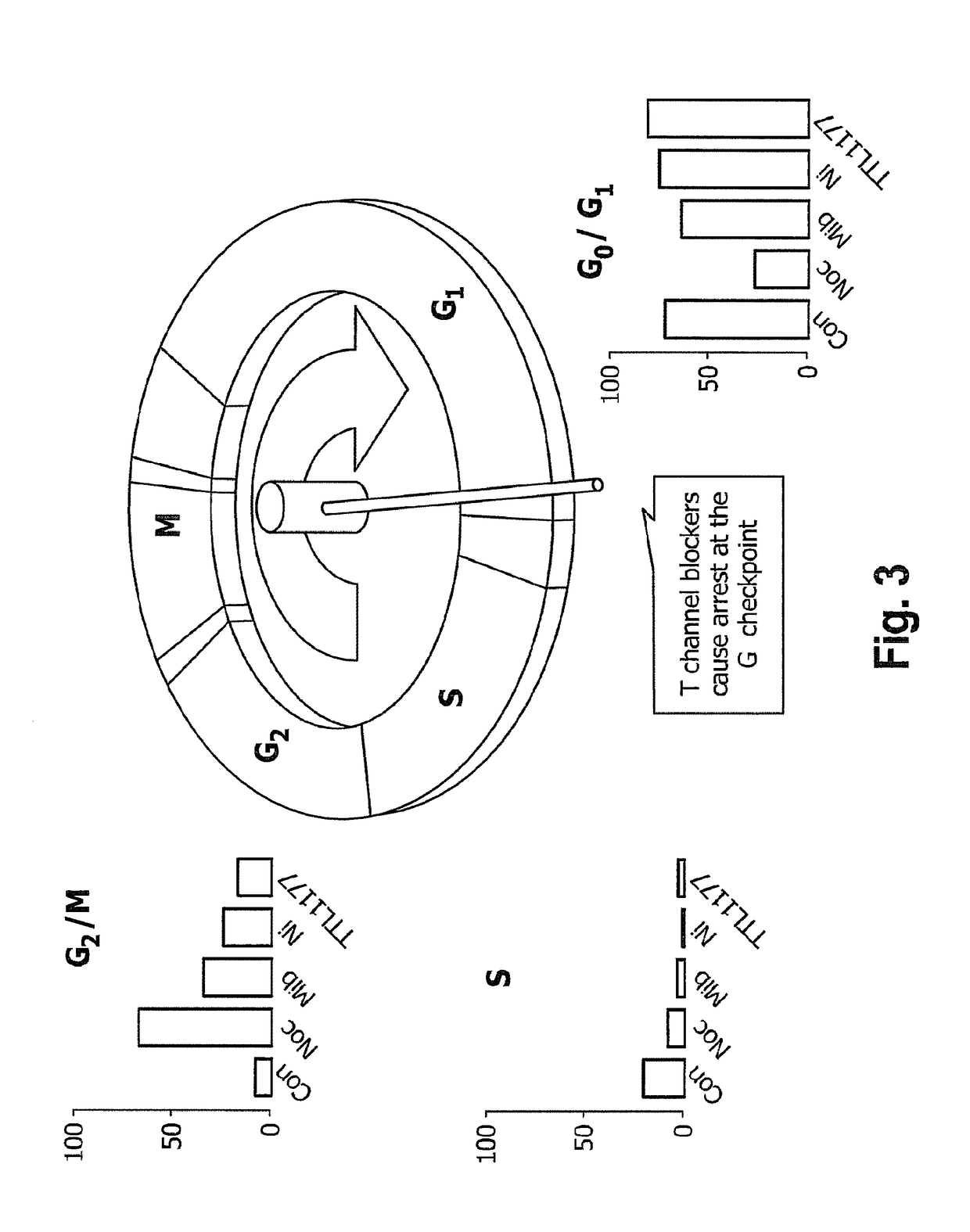
Interlaced Method for Treating Cancer or a Precancerous Condition
InactiveUS20180228822A1Effectively slow and stop progressionRaise the ratioHeavy metal active ingredientsMetabolism disorderDiseaseT-type calcium channel
The present invention provides a method for treating a disease or condition in a mammal which comprises the steps of; administering a therapeutically effective amount of a T type calcium channel inhibitor to effectively slow or stop progression of eukaryotic cells through the S, G2 and M phases of the cell cycle to increase the proportion of the eukaryotic cells in the G1 phase, stopping administration of the T type calcium channel inhibitor for a period of time, and administering a dosage selected from the group consisting of a dosage of at least one chemotherapeutic agent, a dosage of radiation, and combinations thereof, to kill the proportion of eukaryotic cells progressing past the G1 phase of the cell cycle after the stopping of the administration of the T type calcium channel inhibitor.
Owner:CAVION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com