In-vitro assay method for thrombin-activatable fibrinolysis inhibitor (TAFI) content
An in vitro detection and content technology, applied in measurement devices, preparation of test samples, instruments, etc., can solve the problems of expensive equipment, long detection time, low detection limit, etc., and achieve simple operation, low detection limit, diagnosis sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] 2. Preparation of coating antibody and enzyme-labeled antibody
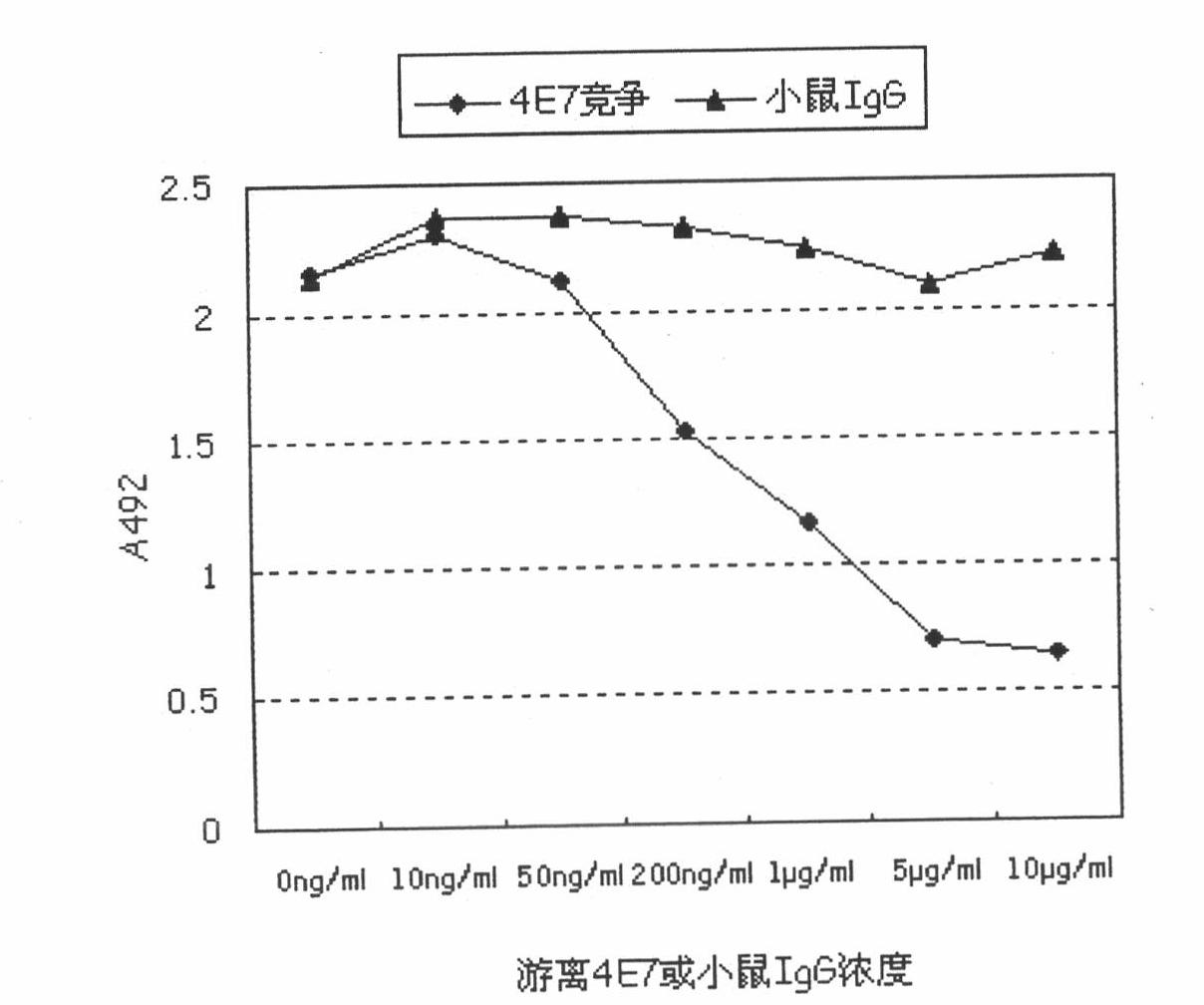
[0047] Coating antibody and enzyme-labeled antibody are mouse anti-human monoclonal antibodies prepared against recombinant human TAFI antigen, and the mouse anti-human monoclonal antibodies include 3B1 and 4E7.
[0048] Hybridoma preparation: use purified human plasma TAFI as the immunogen, and immunize BALB / c mice according to conventional methods; take the splenocytes and fuse them with SP2 / 0 myeloma cells; screen the hybridoma cells, and positive wells are limited to 3 times Cloning by dilution method, and finally obtained two hybridoma cell lines that continuously and stably secrete anti-human TAFI monoclonal antibody, named 3B1 and 4E7 respectively;
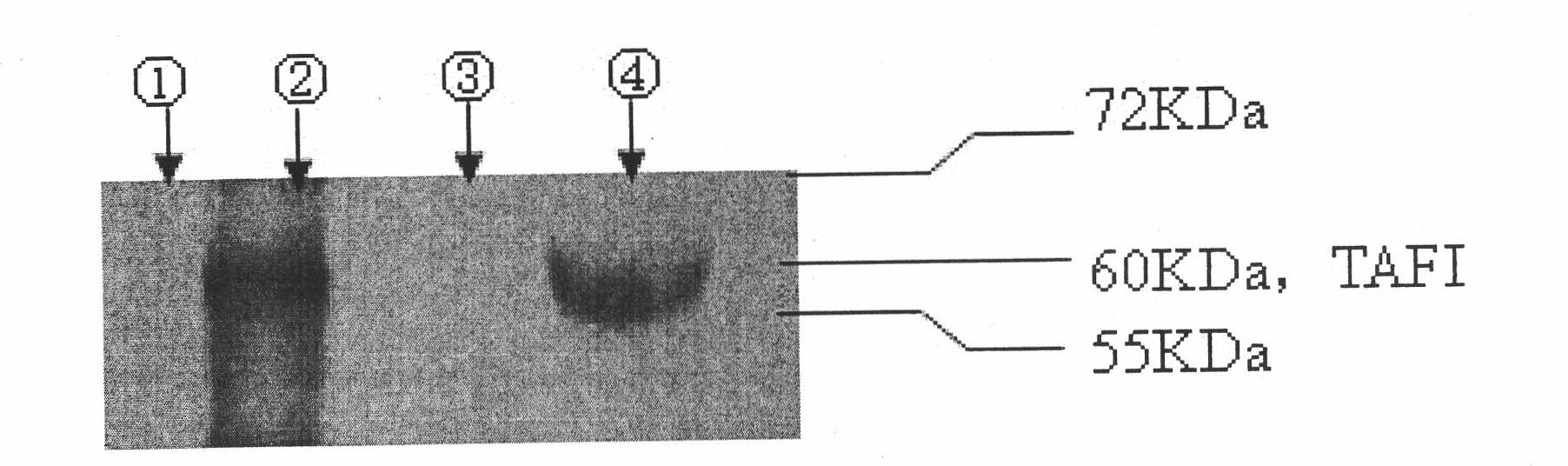
[0049] Antibody identification: According to the instructions of Roche’s antibody subclass kit, the types of the two monoclonal antibodies were determined to be IgG1, κ; using simulated plasma as a control, Western blot and ELISA analysis showed that th...
Embodiment 1
[0078] Taking human plasma as an example, the method of the present invention is used to measure the TAFI content of 58 patients' plasma samples diagnosed as myocardial infarction and 30 healthy plasma samples. The specific operation steps are as follows:
[0079] Step 1 Collect the anticoagulated blood and immediately centrifuge it at 3000 rpm for 10 minutes at 4°C within 30 minutes, take the supernatant as the sample to be tested, and store it at -20°C.
[0080] Step 2: Use an in vitro detection kit to measure the TAFI content of the sample to be tested.
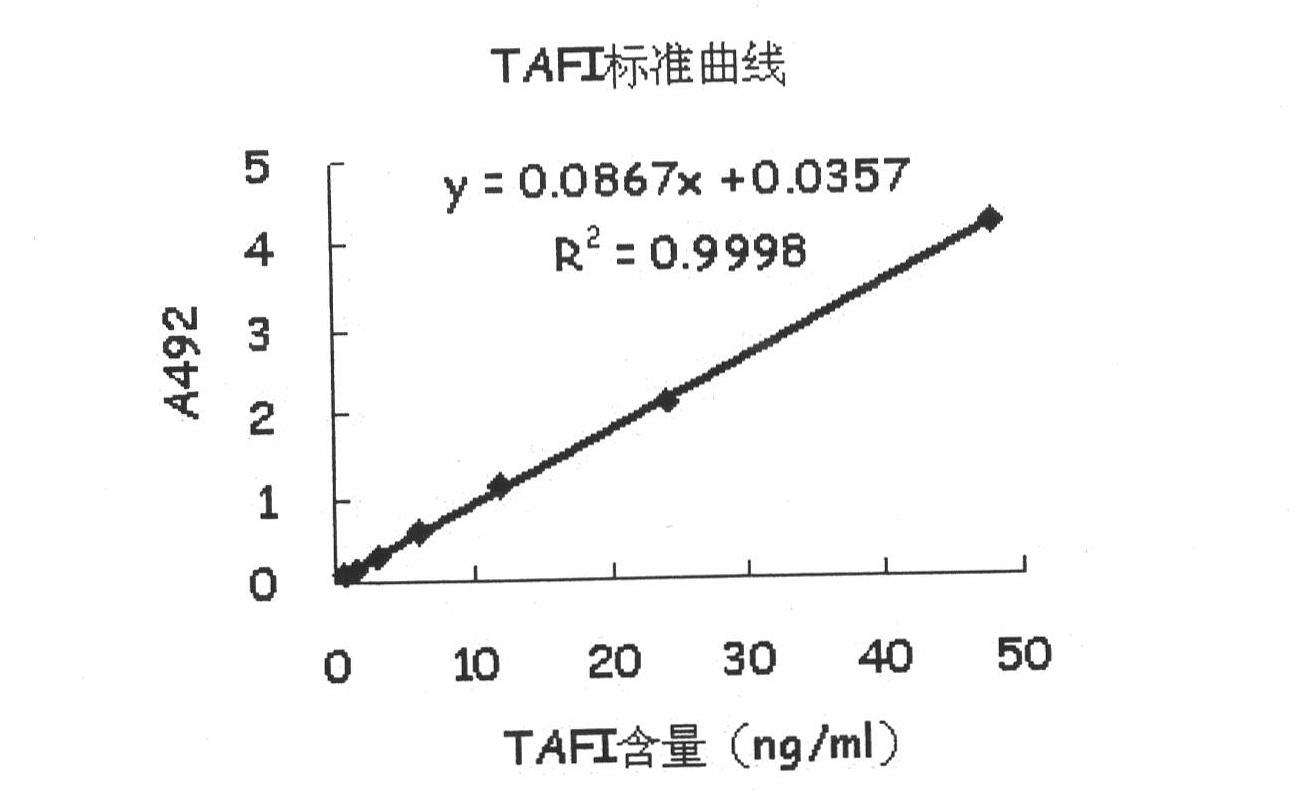
[0081] The contents of the in vitro detection kit used include: a microtiter plate coated with an antibody that captures the TAFI antigen of the sample to be tested and forms an antigen-antibody complex on the solid phase, a sample diluent for diluting the sample to be tested, and used for making calculations The TAFI standard of the standard curve of the TAFI antigen content in the sample to be tested is used to wash awa...
Embodiment 2
[0095] The method of the invention can also measure the TAFI content in human urine. Collect human morning urine, centrifuge at 1500 rpm for 5 minutes at 4°C within 30 minutes, and take the supernatant as the sample to be tested. Dilute the sample to be tested 100 times with the sample diluent. Subsequent steps are the same as in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com