Primers and method for detecting polymorphic hotspot mutation condition of DOCK2 gene
A technology of polymorphic hotspots and sequencing primers, applied in the fields of life sciences and biology, can solve the problems of lack of resistance, deficiency, and inability to synthesize immunoglobulins, etc., and achieve the effect of difficult detection, high cost, and reduced cost and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
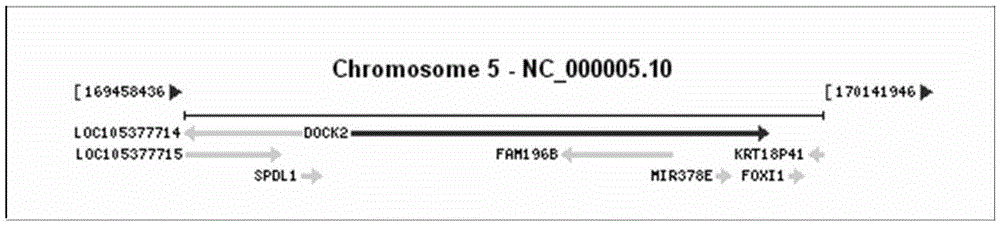
[0078] A primer for detecting polymorphic mutation sites of the DOCK2 gene, the design of the primers is specific amplification primers designed for DOCK2 mutation hotspots, including:
[0079] Primers for amplifying DOCK2 gene sequences containing exons 6, 22, 33, 37, 40, and 44, the base sequences of which are:
[0080] DOCK2-1F: TGTAAAACGACGGCCAGTTTATGGGTTGTTTCTTGTCTCCT
[0081] DOCK2-1R: AACAGCTATGACCATGCCATCGGAAGTAGGTTAAATGAG
[0082] DOCK2-2F: TGTAAAACGACGGCCAGTCTTTAACCTTTGATTGAATGCCT
[0083] DOCK2-2R: AACAGCTATGACCATGTAGGTTGGATTCTTGAGCTGTTT
[0084] DOCK2-3F: TGTAAAACGACGGCCAGTCGTCGCCGAACTGTTTTAT
[0085] DOCK2-3R: AACAGCTATGACCATGGAGAAAGAGGCTAAGCAAATACA
[0086] DOCK2-4F: TGTAAAACGACGGCCAGTGTTATCCAGGGAGGAGGACTTT
[0087] DOCK2-4R: AACAGCTATGACCATGCTCTTCAACTTGCTGTGAGGC
[0088] DOCK2-5F: TGTAAAACGACGGCCAGTGCTTACTGGCTGCTTTACGAG
[0089] DOCK2-5R: AACAGCTATGACCATGTATGATGAGGGCTATTGACTGG
[0090] DOCK2-6F: TGTAAAACGACGGCCAGTTTGCAATATTTATTCTGCTTTCG
[0091] DOCK2-6...
Embodiment 2
[0100] Operation process of blood / cell / tissue genomic DNA extraction kit (Tiangen Biology):
[0101] (1) Extract tissue DNA from blood: 1) Extract 300 μl of blood and add 900 μl of erythrocyte lysate, mix by inverting, and place at room temperature for 5 minutes, during which time, invert and mix several times. Centrifuge at 12,000rpm for 1min, suck off the supernatant, leave the white blood cell pellet, add 200μl buffer GA, shake until thoroughly mixed. 2) Add 20 μl proteinase K solution and mix well. 3) Add 200 μl of buffer GB, mix thoroughly by inversion, place at 70°C for 10 minutes, the solution should become clear, and briefly centrifuge to remove water droplets on the inner wall of the tube cap. 4) Add 200 μl of absolute ethanol, vortex and mix well for 15 seconds. At this time, flocculent precipitates may appear. Briefly centrifuge to remove water droplets on the inner wall of the tube cap. 5) Add the solution and flocculent precipitate obtained in the previous step ...
Embodiment 3
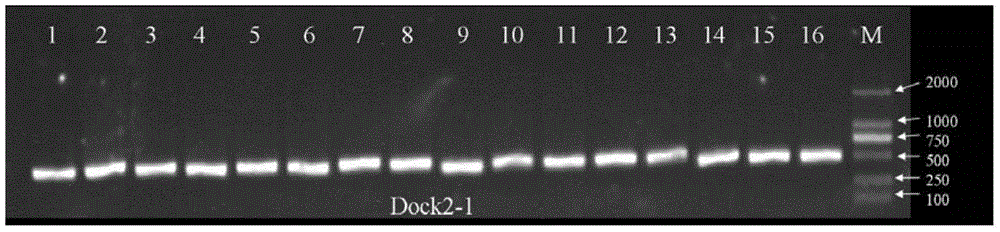
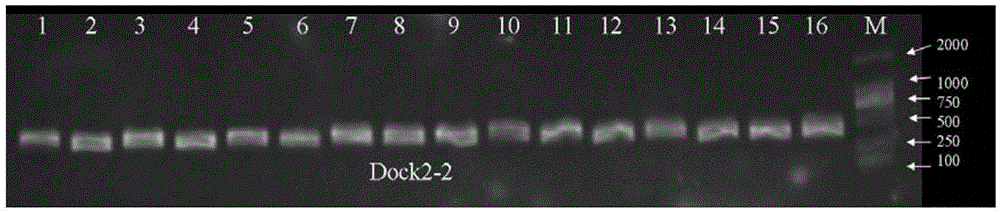
[0128] 16 cases of clinical samples (sample number 1-16) were taken according to the reagents and methods of Examples 1 and 2 to extract genome, prepare reagents, amplify and sequence. Add 1 μl of each sample to the detection system PCR reaction solution. Electrophoresis results such as figure 2 , 3 , 4, 5, 6, and 7, indicating that the primers DOCK2-1F / R, DOCK2-2F / R, DOCK2-3F / R, DOCK2-4F / R, DOCK2-5F / R, DOCK2-6F of the present invention / R can effectively amplify blood samples with a single band.
[0129] The test results of samples 1, 2, 3, 4, 5, and 6 are as follows Figure 8 , 9 , 10, 11, 12, and 13:
[0130] Figure 8 It shows the wild-type sequencing screenshot of DOCK exon 26 of sample 1, indicating that exon 6 of sample 1 is not mutated.
[0131] Figure 9 It shows the wild-type sequencing screenshot of exon 222 of DOCK of sample 2, indicating that exon 22 of sample 2 is not mutated.
[0132] Figure 10 It shows the wild-type sequencing screenshot of DOCK233 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com