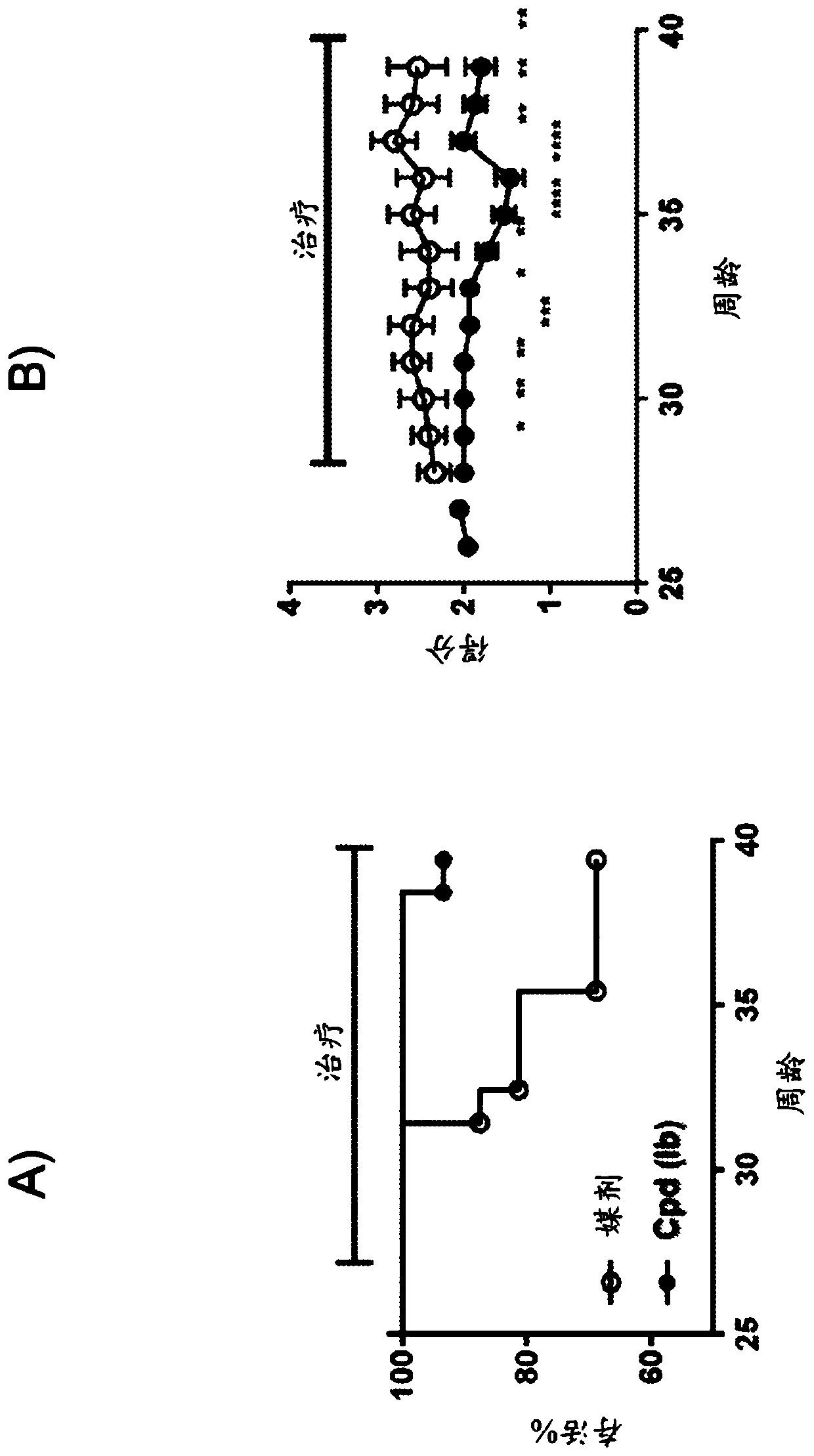
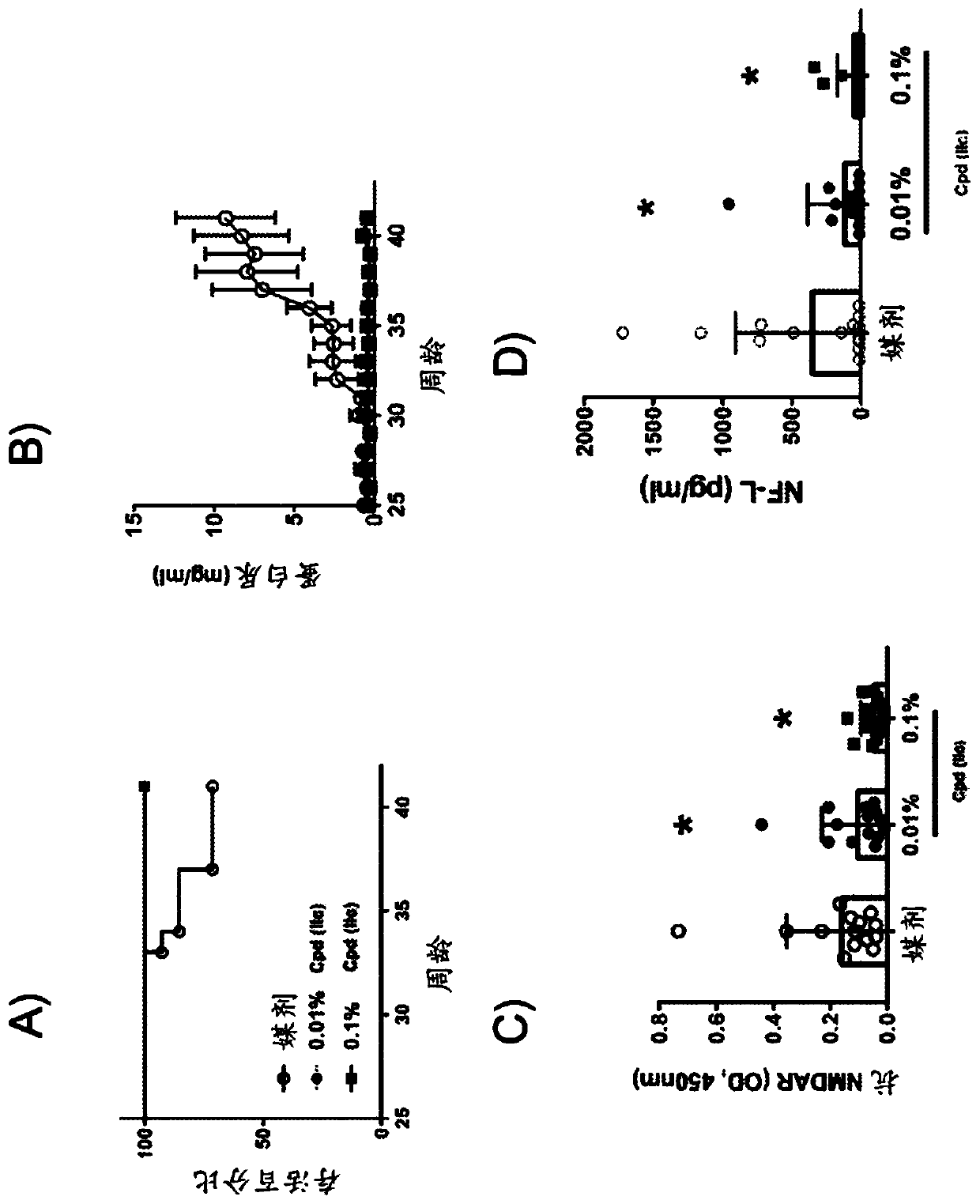
Pyrazolo piperidine and pyrazolo pyrimidine derivatives for treatment of neuropsychiatric systemic lupus erythematosus
A technology of drugs and compounds, applied in the field of pyrazolopiperidine derivatives and pyrazolopyrimidine derivatives for the treatment of neuropsychiatric systemic lupus erythematosus, can solve the problems of high medical demand, no NPSLE treatment method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0157] Preparation of compounds for use in the present invention:
[0158] The synthesis of compounds of formula (I) and formula (II) has been described in US Pat. No. 9,126,999 and PCT application WO 2018 / 047081, respectively. Accordingly, both US 9,126,999 and WO 2018 / 047081 are incorporated by reference.
[0159] Existing Technology and Significance:
[0160] Recently, anti-interferon receptor type 1 (IFNAR) antibodies have shown efficacy in Ph2b studies in lupus patients (published by R. Furie et al., Arthritis and Rheumatology, 69, 376-386, 2017 ). Blockade of IFNAR also reduced the autoimmune phenotype in the lupus 564Igi murine model. In this model, follicular dendritic cells (FDCs) have been identified as the main source of IFNα: autoantibodies and nucleolar immune complexes elicit IFNα from FDCs via the endosomal receptor TLR7 (by A. Das et al., Immunity [Immunology] 46, 106-119, published in 2017).
[0161] In a related study in the 564Igi model, anti-IFNAR trea...
Embodiment 1a
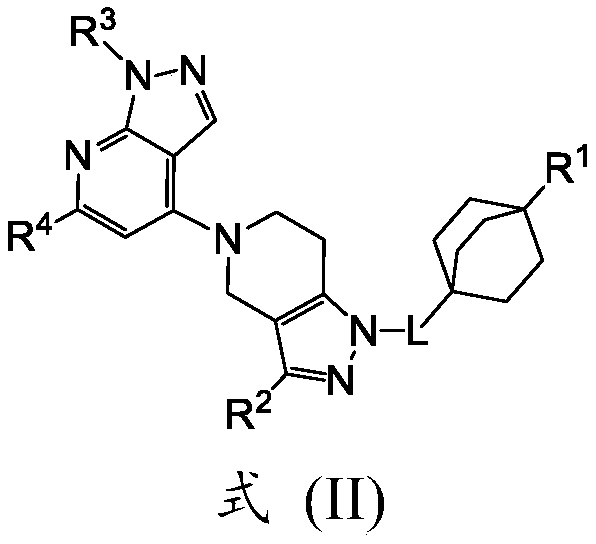
[0205] Embodiment 1a. A method of treating and / or preventing NPSLE comprising administering to a patient in need thereof an effective amount of a compound of formula (II):
[0206]
[0207] in
[0208] L is -CH 2 -or-CH 2 CH 2 -;
[0209] R 1 is -NHC(=O)R 6 , -NHC(=O)(CH 2 ) n R 6 , -NHC(=O)(CH 2 ) m NHR 5 , -NHC(=O)(CH 2 ) m N(R 5 ) 2 , -NHC(=O)(CHR 7 ) m NHR 5 , -NHC(=O)(CH 2 ) m NH 2 , -NHC(=O)(CH 2 ) n OR 7 , -NHC(=O)OR 7 , -NHC(=O)(CHR 7 ) n R 6 , -NHC(=O)(CHR 7 ) n N(R 8 ) 2 , -NHC(=O)(CHR 7 ) n NHR 8 , -NR 7 C(=O)OR 11 , -NHC(=O)(CH 2 ) n N(CD 3 ) 2 , -NR 7 C(=O)R 5 , -NR 7 C(=O)(CH 2 ) n R 5 , -NR 7 C(=O)OR 5 , -NHS(=O) 2 R 5 , -NHC(=O)(CH 2 ) n NR 7 C(=O)R 5 , or -NHC(=O)(CH 2 ) n NR 7 S(=O) 2 R 5 ;
[0210] R 2 is H, C 1 -C 6 Alkyl or C 1 -C 6 haloalkyl;
[0211] R 3 is H, C 1 -C 6 Alkyl or -CD 3 ;
[0212] R 4 is H, NH 2 , C 1 -C 6 Alkyl or halogenated;
[0213] each R 5 independently se...
Embodiment 2a
[0220] Embodiment 2a. The method of Embodiment 1a, wherein the compound is a compound of Formula (IIa):
[0221]
[0222] in
[0223] R 2 is H, C 1 -C 6 Alkyl or C 1 -C 6 Haloalkyl;
[0224] R 3 is H, C 1 -C 6 Alkyl or -CD 3 ;
[0225] R 4 is H, NH 2 、C 1 -C 6 Alkyl or halo;
[0226] R 6 is C 3 -C 6 Cycloalkyl or have independently selected from N, NH, N (C 1 -C 6 Alkyl) and 1 to 2 ring members of O, unsubstituted or replaced by 1-2 R 9 4-6 membered heterocycloalkyl group substituted;
[0227] each R 9 independently selected from C 1 -C 6 Alkyl, hydroxyl, halo and C substituted by 1 to 3 -OH 1 -C 6 alkyl; or a pharmaceutically acceptable salt thereof.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com