A variable region sequence of a specific anti-thiamethoxam antibody and its recombinant full-length antibody
A full-length antibody and variable region technology, applied in the field of genetic engineering, can solve problems such as differences between antibody batches, easy loss of effective genes, and inability of cell lines to produce effective antibodies, achieving high selectivity, high sensitivity, and guaranteed recognition active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to enable those skilled in the art to better understand the solutions of the present invention, the technical solutions in the embodiments of the invention will be clearly and completely described below in conjunction with the drawings in the embodiments of the present invention. Obviously, the described embodiments are only It is a part of embodiments of the present invention, but not all embodiments. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative work should belong to the protection scope of the present invention. If there is no special description, the experimental methods in the embodiments are all for the conventional method.
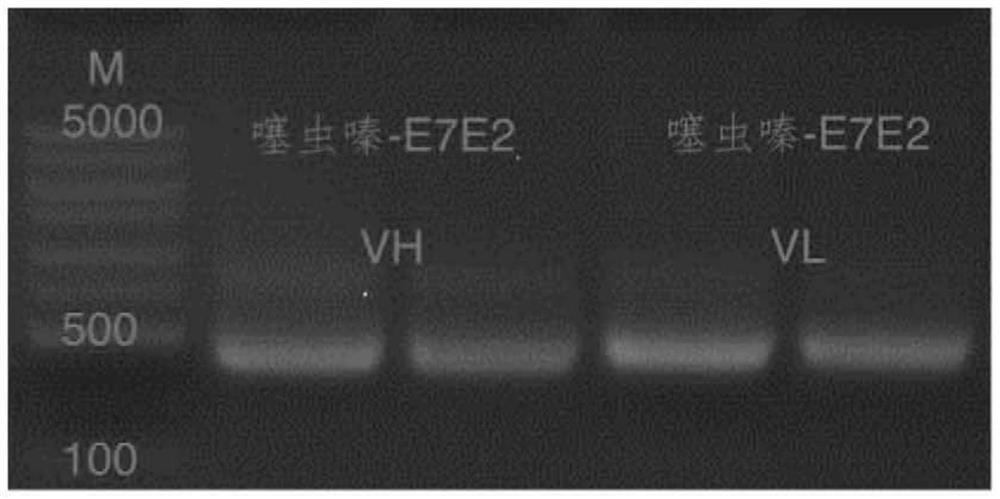
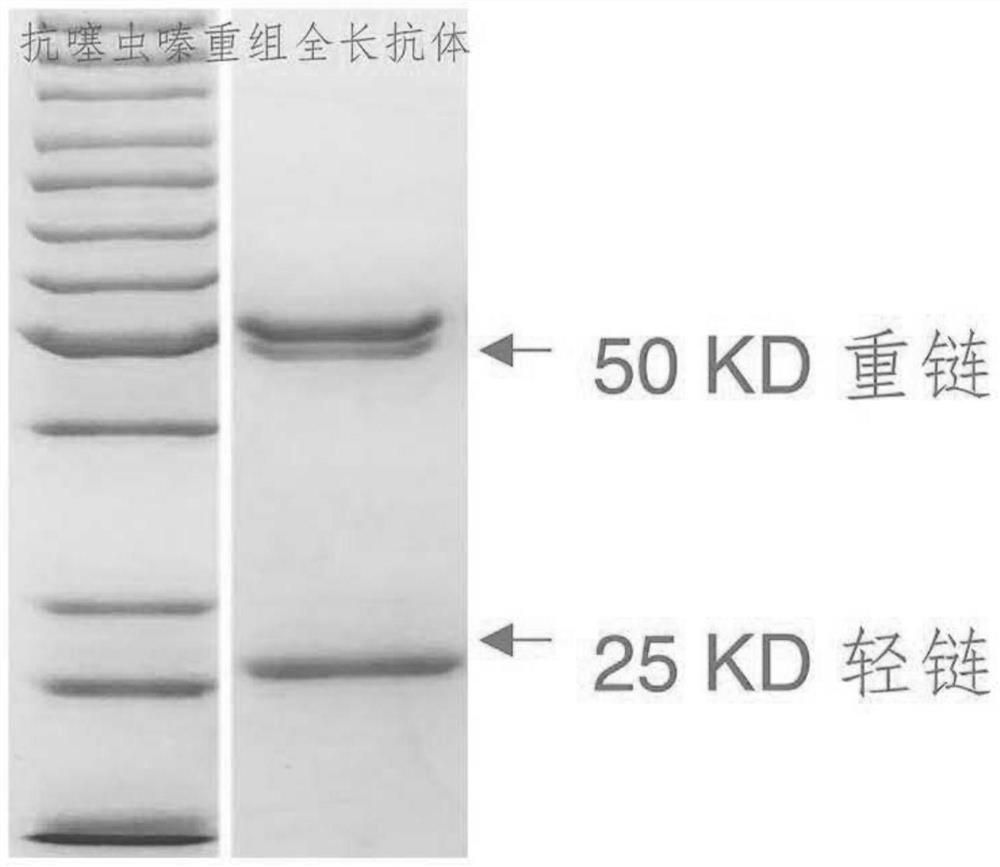
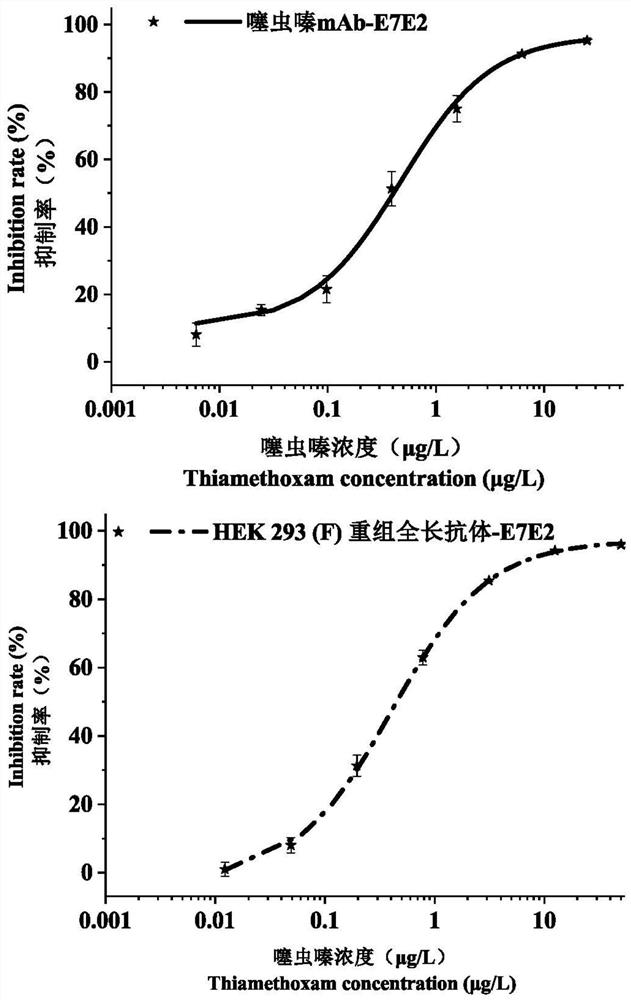
[0023] The anti-thiamethoxam recombinant full-length antibody involved in the present invention includes a heavy chain constant region, a heavy chain variable region, a light chain constant region and a light chain variable region, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com