Method of treatment with tradipitant
A Pittan, Kawachi technology, applied in the field of NK-1 antagonists, can solve the problems of uncontrollable opioid use, personal relationship or financial negative impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
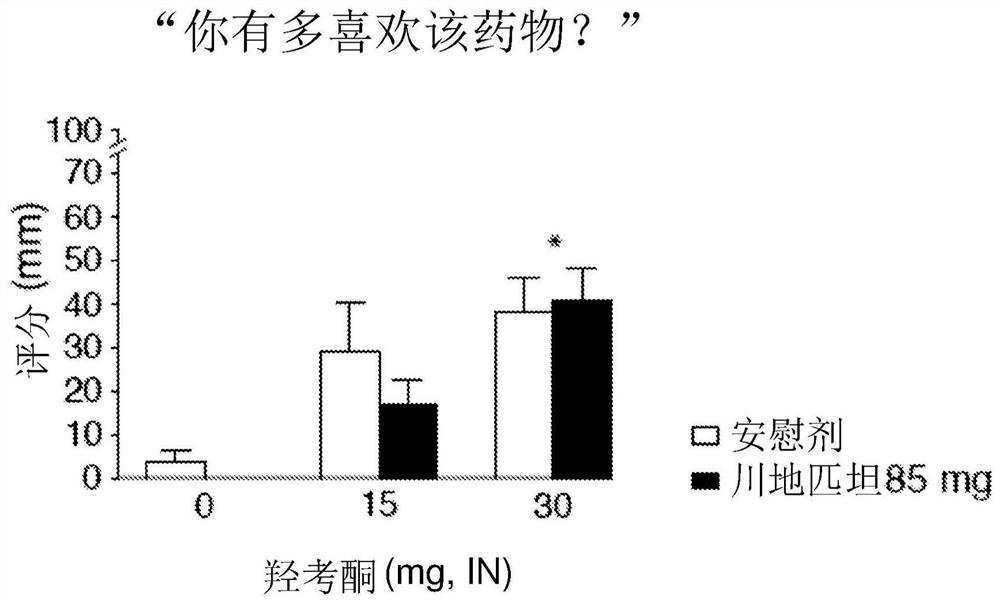
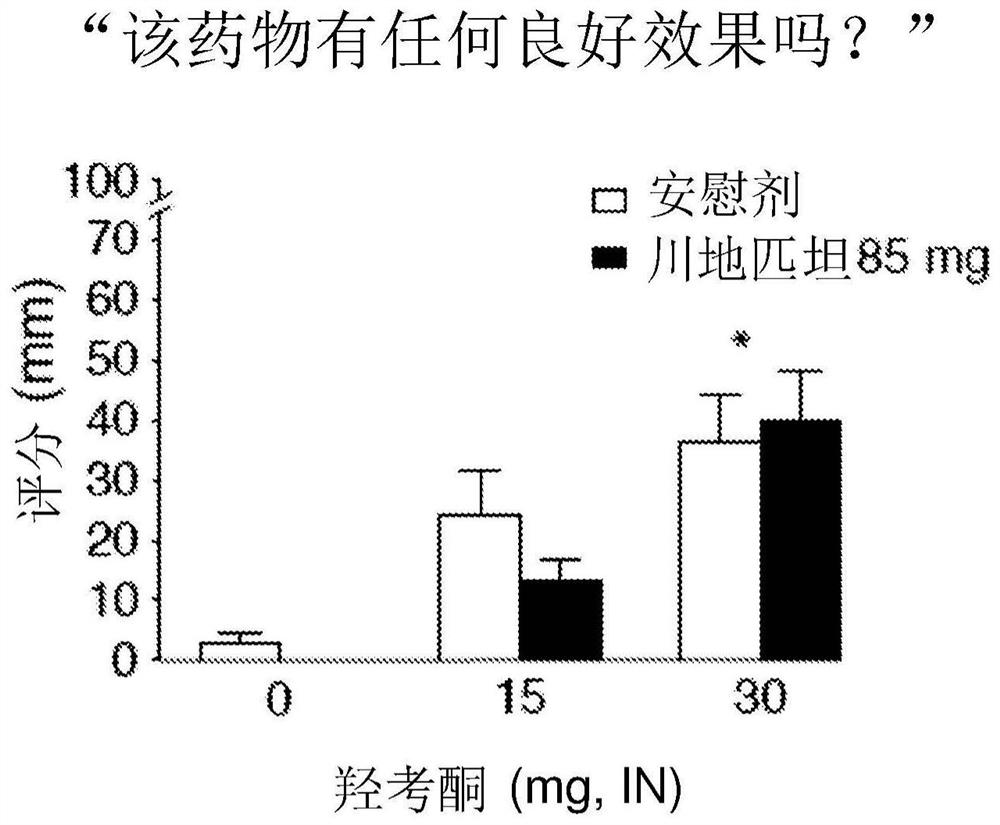
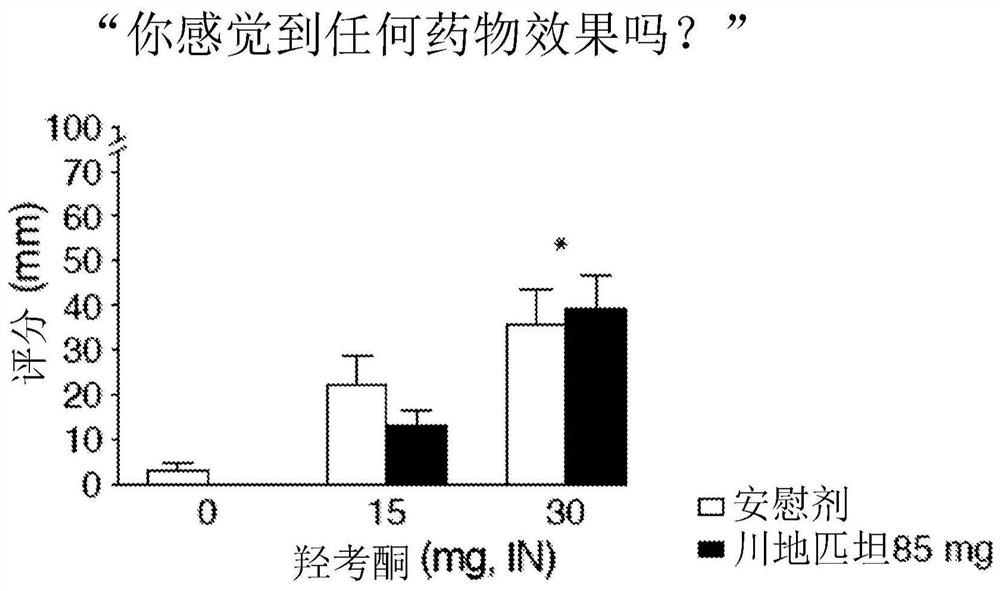
[0032] A double-blind, approximately 6-week study in inpatients with a within-subjects crossover design examining the effect of maintaining oxycodone response with sendipitant compared with placebo. Study subjects included healthy adults who reported regular illicit opioid misuse and a history of intranasal opioid use without physical dependence. Participant characteristics are provided in Table 1 below.
[0033]
[0034] Subjects received placebo or sendipitant (85 mg, orally (p.o.), twice daily) on study days 3-17, with a washout period on study days 18-23, and then on study days 24-24. Placebo or sendipitant (85 mg, po., twice daily) was administered for 39 days. In this study, sendipitant / placebo administration was balanced among subjects. On Study Days 3, 18, 24, and 39, subjects participated in a challenge phase (Day 1 and Steady State) in which they received 0, 5, 10, or 20 mg intranasal (IN) oxycodone at 1-hour intervals , subsequently evaluating the analgesic ef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com