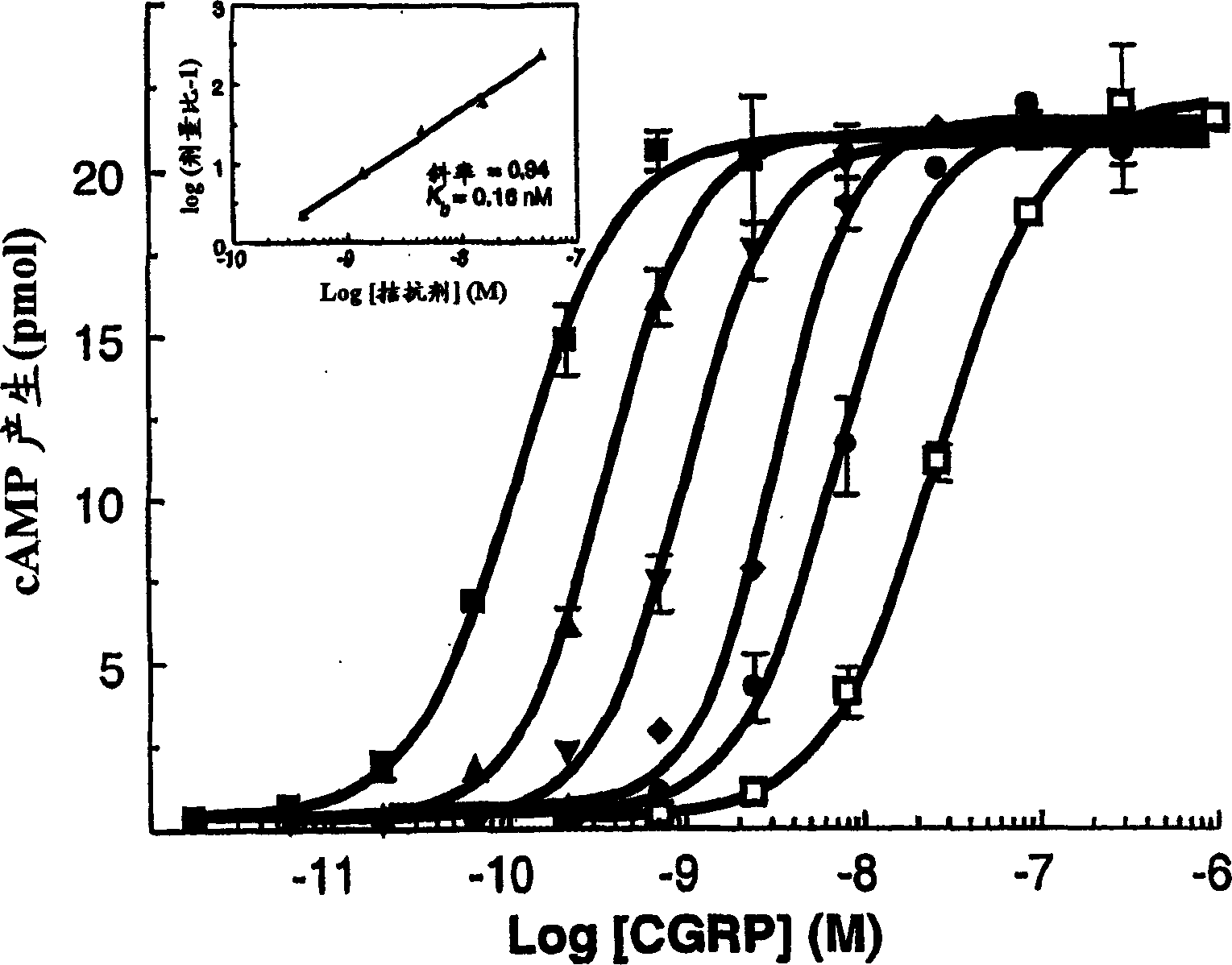
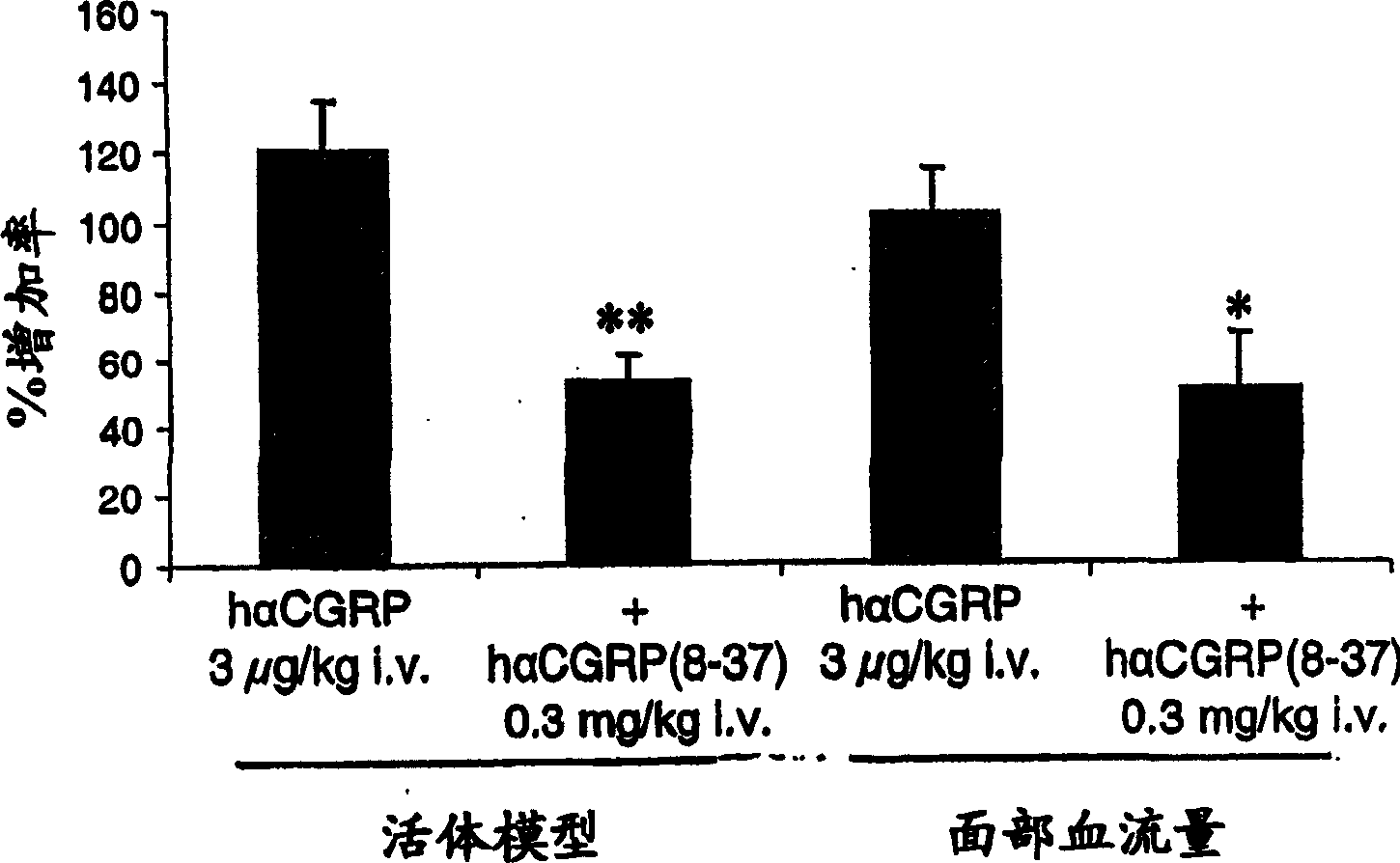
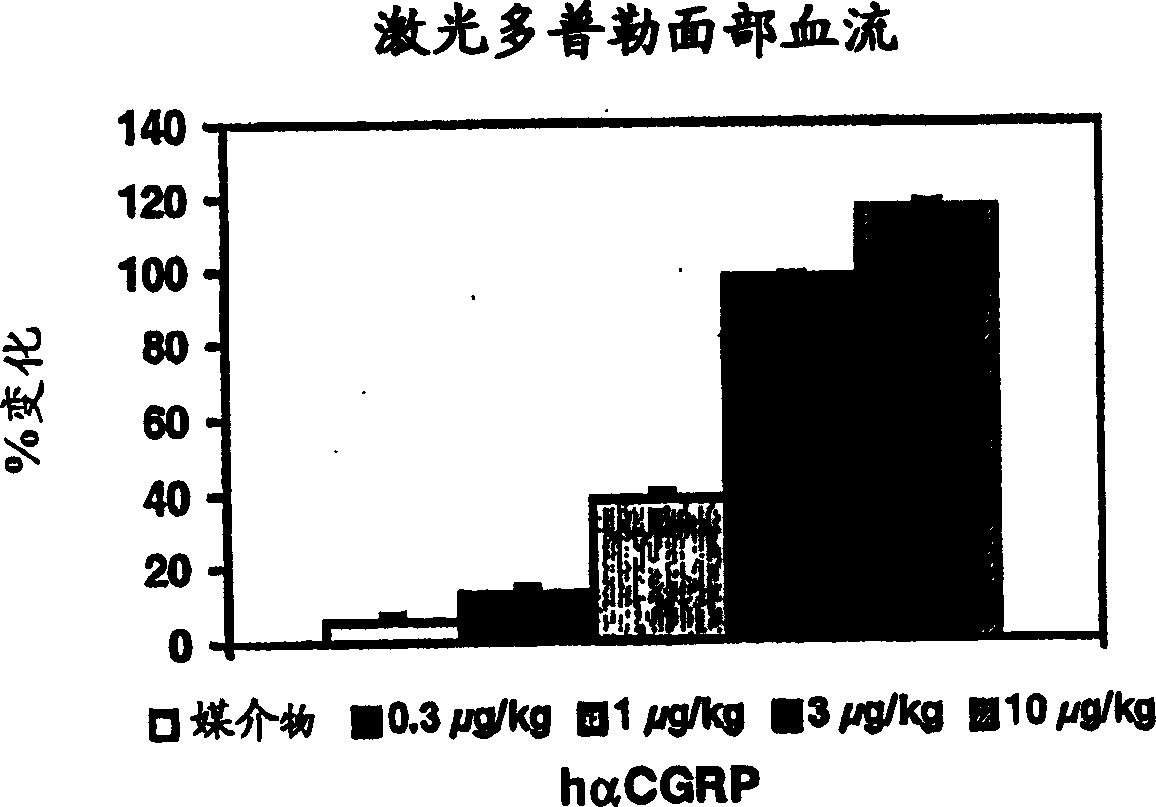
Calcitonin gene related peptide receptor antagonists
An alkyl, phenyl technology, applied in the field of calcitonin gene-related peptide receptor antagonists, which can solve the problem of not showing the disruptive effect of arterial dilatation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0312] HNR 1 R 2 and the preparation of formula VIII amine
[0313] Formula VIII and HNR 1 R 2 Amines are available commercially or may be prepared by literature methods or methods described herein.
[0314]
[0315] Preparation of Amino Acids of Formula II and V
[0316]
[0317] Amino acids of Formula II and V may be commercially available or prepared as described in Scheme 4.
[0318] Scheme 4. Synthesis of Formula II and Formula V Compounds
[0319]
[0320] The synthesis described in Scheme 4 starts with an aldehyde of formula IX which is reacted with a phosphonoglycinate of formula X via Wadsworth-Emmons coupling. Compounds of formula X are deprotonated with a base such as diazabicycloundecene or tetramethylguanidine or other organic or inorganic bases well known in the art. Reduction of the double bond of the resulting compound of formula XI affords a compound of formula XII. Reduction can be carried out to give the racemate, or by using a stereoselecti...
Embodiment 1
[0485] (±)-3-(1H-indazol-5-yl)-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine- 1-Carbonyl]-amino}-propionic acid
[0486]
[0487] 5-(2-methoxycarbonyl-2-{[4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carbonyl]-amino }-Ethyl)-indazole-1-carboxylic acid tert-butyl ester (168 mg, 0.29 mmol) dissolved in THF (5 mL) in methanol (5 mL) was cooled to 0°C. Lithium hydroxide monohydrate (49mg, 2.04mmol) in water (5ml) was added. The reaction mixture was stirred at 0°C for 6 hours, then placed in the refrigerator for an additional 16 hours. The solvent was removed in vacuo and the residue was dissolved in water (15 mL). The pH of the aqueous solution was adjusted to about 1 with 1N hydrochloric acid. The precipitated white solid was collected by filtration. The solid was dried in vacuo to give the title compound (108 mg, 80%).
[0488] 1 H-NMR (DMSO-d 6 , 300MHz) δ12.94(bs, 1H), 9.19(s, 1H), 8.01(s, 1H), 7.61(s, 1H), 7.46(d, J=8.4Hz, 1H), 7.28(dd, J =8.5, 1...
Embodiment 2
[0543] (R)-4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid [2-[1,4′]bipiperidine-1 '-yl-1-(1H-indazol-5-ylmethyl)-2-oxoethyl]-amide ()
[0544]
[0545] At 80°C, (R)-4-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidine-1-carboxylic acid {2-[1,4'] Bipiperidin-1'-yl-2-oxo-1-[1-(2-trimethylsilyl-ethanesulfonyl)-1H-indazol-5-ylmethyl]-ethyl}-amide (568 mg, 0.73 mmol) and cesium fluoride (1.11 g, 7.31 mmol) in acetonitrile (50 mL) were heated for 4.5 hours. The reaction mixture was concentrated, and the residue was subjected to flash column chromatography (dichloromethane / methanol / triethylamine, 94:5:1) to obtain 280 mg (63% yield) of the title compound as a white solid, which was determined to be 98.2 by HPLC analysis. %ee, using a ChirocelOD column with 20% B (A = ethanol, B = 0.05% diethylamine in hexane) as eluent (retention time: 9.51 min for the title compound and 15.9 min for the S-enantiomer minute).
[0546] 1 H-NMR (CD 3 OD, 500MHz) δ8.04(s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com