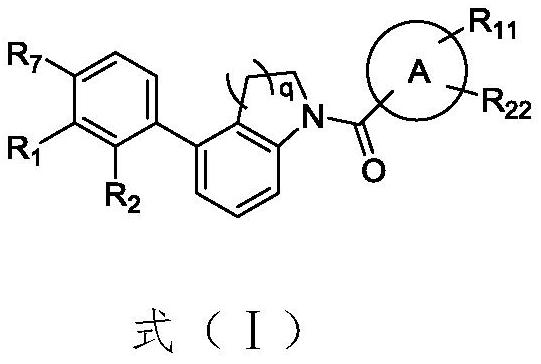
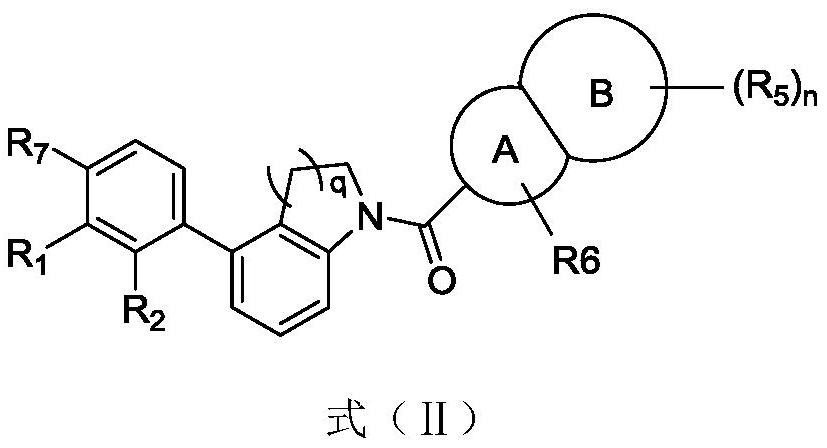
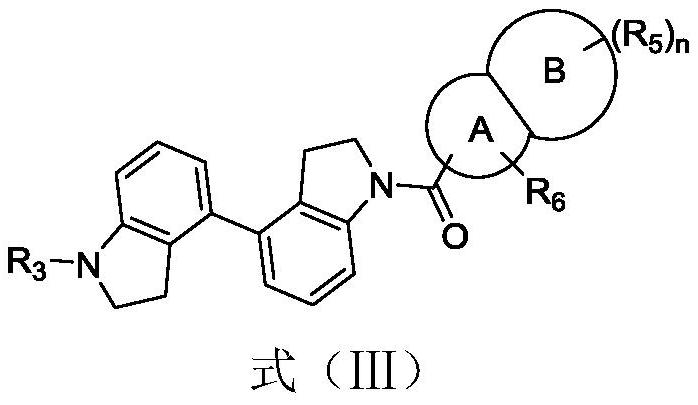
Immunomodulators, compositions and methods thereof
A technology of compounds and chelates, applied in the field of pharmaceutically active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] The synthesis of embodiment 1 compound 1
[0214] 2-(2-(1'-Methyl-[4,4'-diindoline]-1-carbonyl)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H) -yl)acetic acid
[0215]
[0216] To a solution of 4-bromoindole (50 mg) in ACN (20 mL) was added iodomethane (100 mg) and K 2 CO 3 (100mg). The mixture was stirred at 80°C for 12 hours. After the resulting solution was concentrated, the resulting solid was purified by column chromatography to give 4-bromo-1-methylindoline (50 mg).
[0217] To a solution of 4-bromo-1-methylindoline (100 mg) in dioxane (6 mL) was added 4,4,4',4',5,5,5',5'-octamethyl -2,2'-bis(1,3,2-dioxaborinane) (100mg), KOAC (50mg), Pd(dppf)Cl 2 (20mg). The mixture was stirred at 85°C for 12 hours. After the obtained solution was concentrated, the obtained solid was purified by column chromatography to obtain Compound 1-2 (100 mg).
[0218] Compound 1-2 (50mg), compound 4-3 (40mg), K 2 CO 3 (60mg) and Pd(dppf)Cl 2 (10 mg) in 1,4-dioxane (6 mL) and water...
Embodiment 4
[0219] The synthesis of embodiment 4 compound 4
[0220] 2-(2-(4-Phenylindoline-1-carbonyl)-6,7-dihydrothiazolo[5,4-c]pyridin-5(4H)-yl)acetic acid
[0221]
[0222] Step 1: Preparation of tert-butyl 2-(4-bromoindole-1-carbonyl)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H)-acetate
[0223] To a solution of 5-(tert-butoxycarbonyl)-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid (200 mg) in dry dichloromethane was added HATU (200 mg) and DIEA (5 mL). The mixture was stirred for 10 minutes. 4-Bromoindoline was added. The mixture was stirred at room temperature for two hours. After adding 100 mL of EA, washed with brine (4X20 mL). Na for organic phase 2 SO 4 dry. After the resulting solution was concentrated, the resulting solid was purified by column chromatography to give 2-(4-bromoindoline-1-carbonyl)-6,7-dihydrothiazolo[5,4-c]pyridine-5(4H )-acetate (120mg).
[0224] Step 2 Preparation of (4-bromoindol-1-yl)(4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com