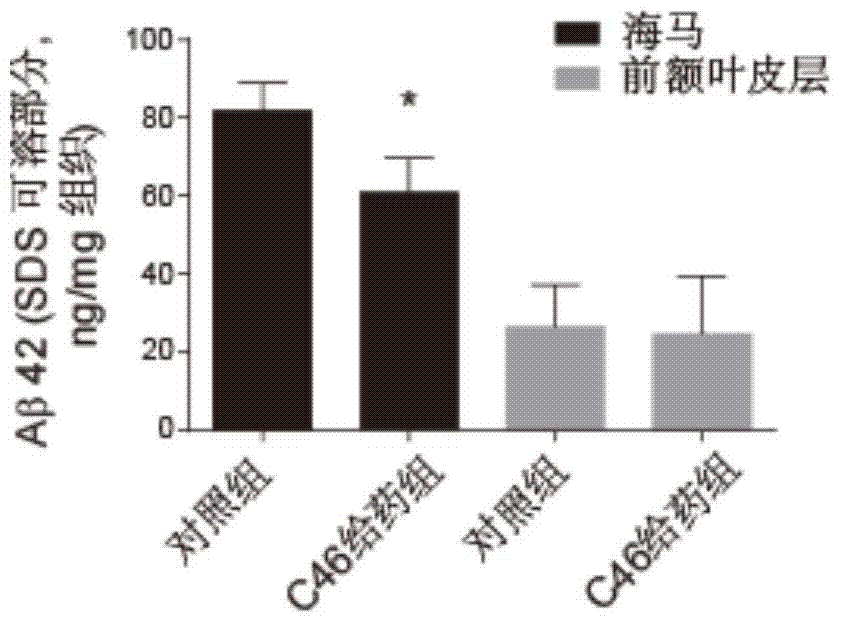
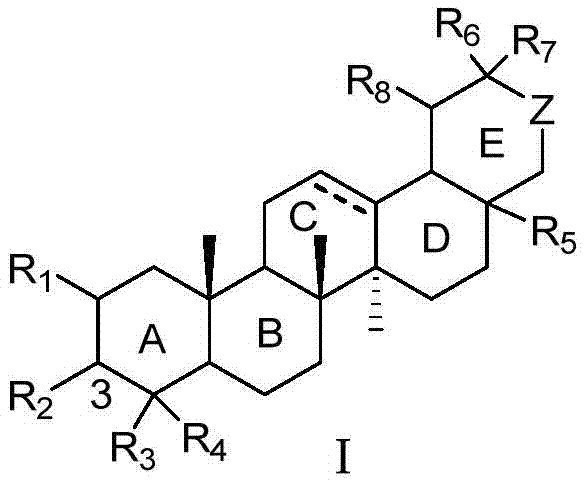
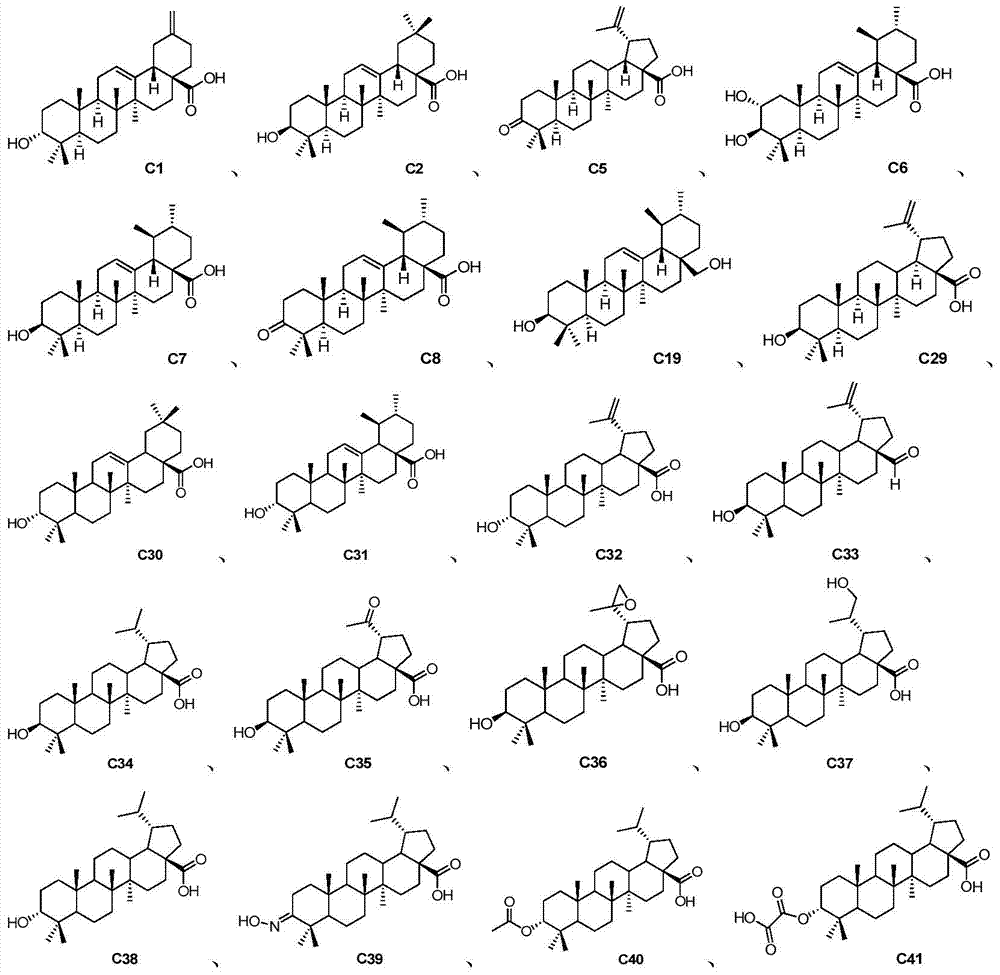
A class of pentacyclic triterpenoids and its use in the preparation of medicines for treating Alzheimer's disease
A technology of pentacyclic triterpenoids and compounds, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0040] Preparation Example 1: Synthesis of Compound C33
[0041]
[0042] (1) Benzyl betulinate
[0043] Dissolve the raw material betulinic acid (4g, 8.76mmol) (purchased from Xi'an Haoxuan Biotechnology Co., Ltd.) in DMF (50mL) at room temperature, add anhydrous potassium carbonate (2.4g, 17.37mmol), and slowly drop it under stirring After adding benzyl chloride (1.2 mL, 10.52 mmol) dropwise, the reaction solution was moved to 50° C. and stirred overnight. The next day, the mixture was cooled to room temperature, diluted with 100 mL of deionized water, extracted with ethyl acetate (2×100 mL), and the combined organic layers were washed with deionized water and saturated brine, dried over sodium sulfate and reduced Pressure distillation gave the desired white solid compound benzyl betulinate (4.62 g), molar yield: 97%. 1 H NMR (300MHz, CDCl 3 )δ7.34(m,5H),5.09(d,1H,J=11.7Hz),5.17(d,1H,J=11.7Hz),4.75(s,1H),4.62(s,1H),3.21- 3.15(m,1H),2.92-2.80(m,1H),2.10-1.90(m,2H),1.87...
preparation Embodiment 2
[0050] Preparation Example 2: Synthesis of Compound C35
[0051]
[0052] Betulinic acid (48mg, 0.11mmol) was dissolved in methanol / dichloromethane (5mL / 5mL), and ozone was passed through at -78°C. TLC showed that the reaction was complete. After the supply of ozone was stopped, the residual ozone was exhausted with oxygen, and the reaction was quenched by adding dimethyl sulfide (25 μL), and the temperature was slowly raised to room temperature, and reacted overnight. The next day, the reaction solution was concentrated under reduced pressure, and the obtained residue was purified by silica gel column chromatography (dichloromethane / methanol=50 / 1, V / V) to obtain compound C35 (21 mg, yield 43.6%). 1 H NMR (300MHz, CDCl 3 ),1.00(s,3H),0.96(s,3H),0.91(s,3H),0.82(s,3H),0.75(s,3H); ESI-MS(m / z):481.3(M+ Na) + (C 29 h 46 o 4 Theoretical value: 458.34).
preparation Embodiment 3
[0053] Preparation Example 3: Synthesis of Compound C41
[0054]
[0055] Compound C42 (12mg, 0.02mmol) was dissolved in ethanol / water (4mL / 1mL) mixed solvent, 2N sodium hydroxide (1mL) was added, and stirred overnight at room temperature. The next day, TLC detected that the reaction was complete. After concentrating the solvent under reduced pressure to remove ethanol, adjust the pH to 3 with 1N hydrochloric acid solution, then extract with ethyl acetate, combine the organic phases and wash with saturated sodium chloride solution, dry the organic phase, and concentrate , the resulting residue was purified by silica gel column chromatography (dichloromethane / methanol=10 / 1, V / V) to obtain compound C41 (6 mg, yield: 50%) as a white solid. 1 H NMR (300MHz, CDCl 3 )δ4.78 (s, 1H), 2.28-2.18 (m, 4H), 1.98-1.15 (m, other alicyclic hydrocarbon protons), 1.01 (s, 3H), 0.94 (s, 3H), 0.92 (s, 3H),0.85(s,3H),0.83(s,3H),0.76(s,3H),0.74(s,3H); ESI-MS(m / z):553.3(M+Na) + (C 32 h 50 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com