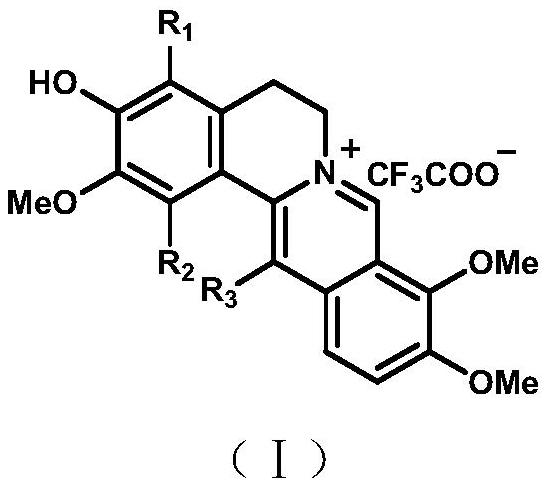
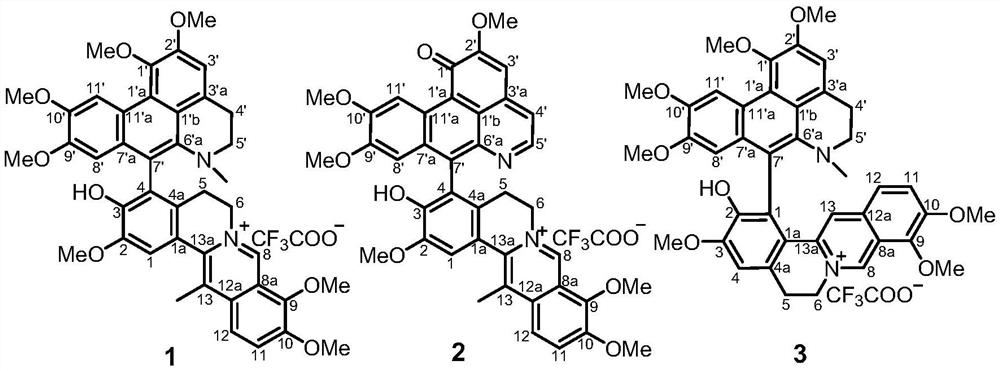
A class of alkaloid dimers and their application in the preparation of pd-1/pd-l1 pathway inhibitors
An alkaloid dimer and compound technology, applied in the field of medicine, can solve the problems of blocking PD-1/PD-L1 interaction, which have not yet been seen, and achieve sustainable industrial production, good research and development prospects, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of Corydalis Corydalis extract includes the following steps:
[0031] (1) Acetic acid processing: 50 kg of tubers of Corydalis yanhusuo W.T. Wang, a traditional Chinese medicine, were soaked in 10L 6% acetic acid solution, and then dried at 40°C.
[0032] (2) Extract: The medicinal materials prepared with acetic acid were crushed and soaked in water, and then extracted by ultrasonic for 3 times, each time using 50 L of water for 1 hour, and the extracts were combined and concentrated under reduced pressure to obtain the plant extract.
Embodiment 2
[0034] The preparation of alkaloid dimer compound comprises the following steps:
[0035] The extract obtained in Example 1 was subjected to macroporous adsorption resin column chromatography, eluted with water, 50% ethanol, and 95% ethanol respectively, and the eluent was concentrated under reduced pressure; the 95% ethanol extract was concentrated to no alcohol, and distilled water was added. 2L, extracted 5 times with an equal volume of ethyl acetate to obtain an ethyl acetate phase and an aqueous phase. The ethyl acetate fraction E is obtained after the organic solvent is recovered under reduced pressure. Sample E was eluted by silica gel column chromatography (dichloromethane-methanol 200:1, 150:1, 100:1, 50:1, 25:1, 10:1, 5:1, 3:1, 0:1) , to get components A-I. Component H was selected and subjected to Sephadex LH-20 column chromatography (petroleum ether-dichloromethane-methanol 5:5:1) to obtain H-2. H-2 was separated by preparative liquid chromatography (Rp C18) to ...
experiment example 1
[0043] Experimental Example 1: Evaluation of PD-1 / PD-L1 Interaction Inhibitory Activity of the Plant Extract of Example 1 of the Invention and the Alkaloid Dimer Compounds Obtained in Example 2
[0044] Experimental principle: Homogeneous Time-resolved Fluorescence (HTRF) uses the chelate and label of Europium with a crypt structure as an energy donor (Donor) and XL665 as an energy acceptor (Acceptor) to form fluorescence resonance energy. Transfer (FRET), the absorbance value at 665nm wavelength increases, and the absorbance value at 620nm wavelength decreases. Two tag antibodies, anti-Tag1-Europium and anti-Tag2-XL665, bind to Tag1-PD-L1 and Tag2-PD1, respectively. When PD-1 / PD-L1 binds, the distance between the two antibodies is just enough for energy Transfer, excitation causes fluorescence resonance energy transfer (FRET), and the absorbance value increases at 665 nm wavelength. When the PD-1 / PD-L1 interaction is interfered by compounds or antibodies, the energy transfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com