Method for synthesizing chlorantraniliprole
A technology for chlorantraniliprole and a synthesis method, which is applied in the legal field of chlorantraniliprole, can solve problems such as poor atom economy, three wastes, and cumbersome treatment process, and achieves the effect of increasing treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
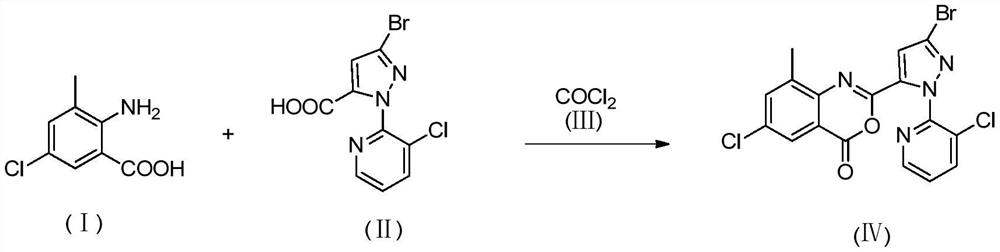
[0024] Step A: 2-(3-Bromo-1-(3-chloro-2-pyridyl)-1H-5-pyrazolyl)-6-chloro-8-methyl-4H-benzo[d][1 ,3] Synthesis of oxazin-4-one
[0025] In a 100ml three-necked flask, configure a condenser, add 45ml of acetonitrile, cool down to 0°C, inject 10g of phosgene, and keep warm at 0-5°C for later use. In a 250ml four-necked flask, add 50ml of acetonitrile, 9g of 3-picoline, 15g of 3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid, 9.2g of 2 -Amino-5-chloro-3-methylbenzoic acid, cooled down to 0°C under stirring, began to add dropwise phosgene acetonitrile solution, controlled the reaction temperature at 0-5°C, after 3h dropwise addition, kept the temperature at room temperature for 1h to obtain The yellow suspension was bubbled with nitrogen for 1 h, and then filtered with suction to obtain a yellow wet product, which was directly used in the next step of synthesis without purification.
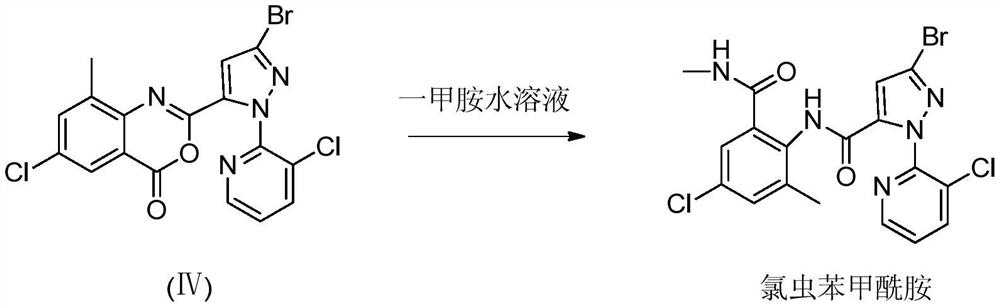
[0026] Step B: the synthesis of chlorantraniliprole
[0027] In a 250ml four-neck ...
Embodiment 2~4
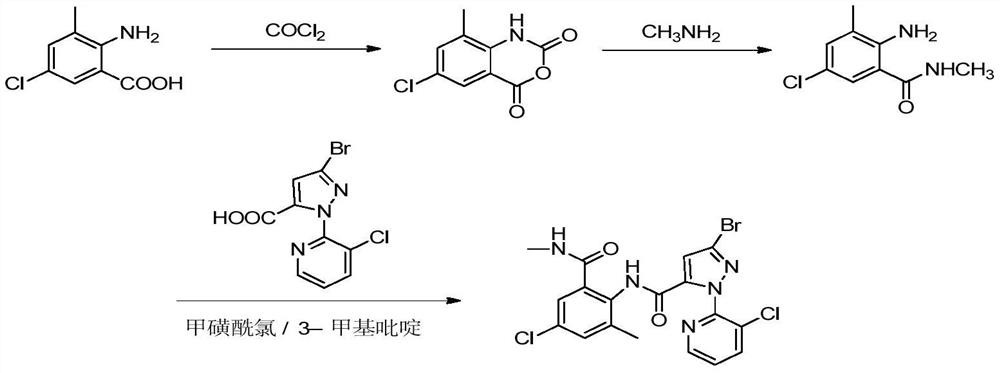
[0029] According to the method of steps A and B in Example 1, different solvents in step A are replaced, and the solvent added is 45ml dissolved phosgene, and the solvent (which is the same solvent as dissolved phosgene) is 50ml mixed organic base, 3-bromo- 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid, 2-amino-5-chloro-3-methylbenzoic acid, reaction conditions and results are as shown in table 1, it can be seen that adopt Acetonitrile had the best effect as a solvent, and the purity and yield of chlorantraniliprole were the highest.
[0030] The reaction conditions and result contrast of table 1 embodiment 2-4
[0031]
Embodiment 5~7
[0033] According to the method of steps A and B in Example 1, adjust the reaction temperature in step A. The reaction conditions and results are shown in Table 2. It can be seen that the reaction temperature is the best when the reaction temperature is 0-5 ° C. The yield of chlorantraniliprole and high purity.
[0034] The reaction condition of table 2 embodiment 5-7 and result contrast
[0035]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap