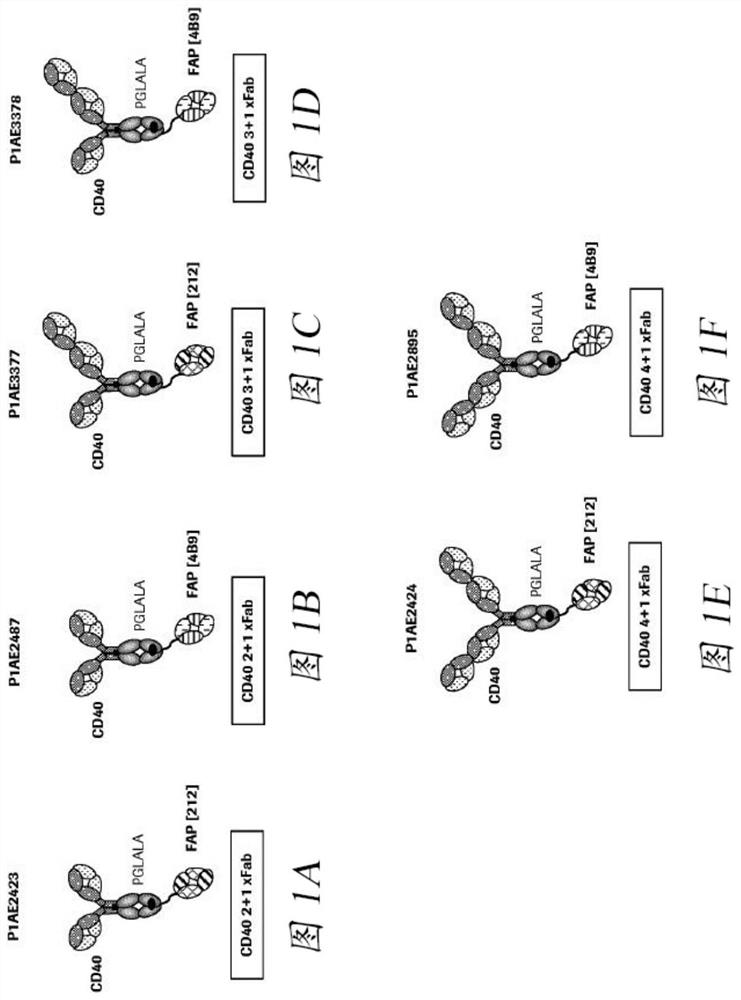
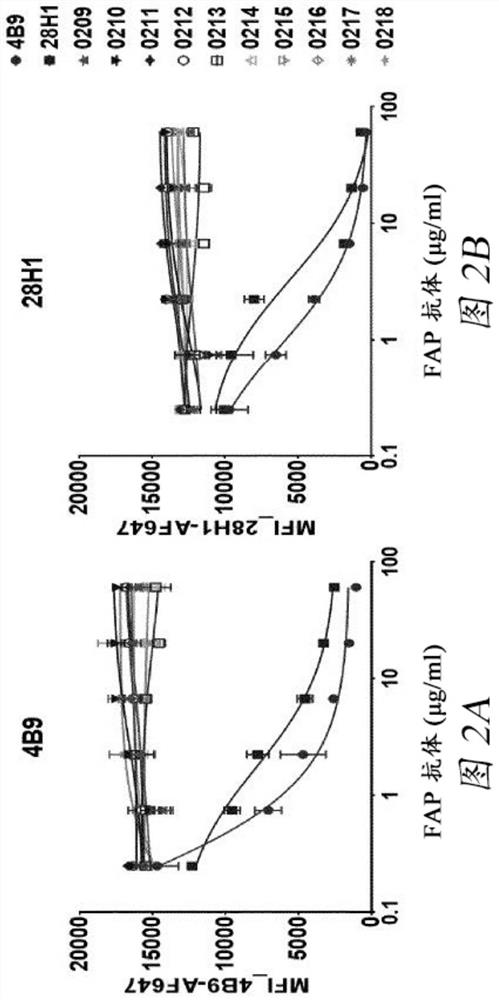
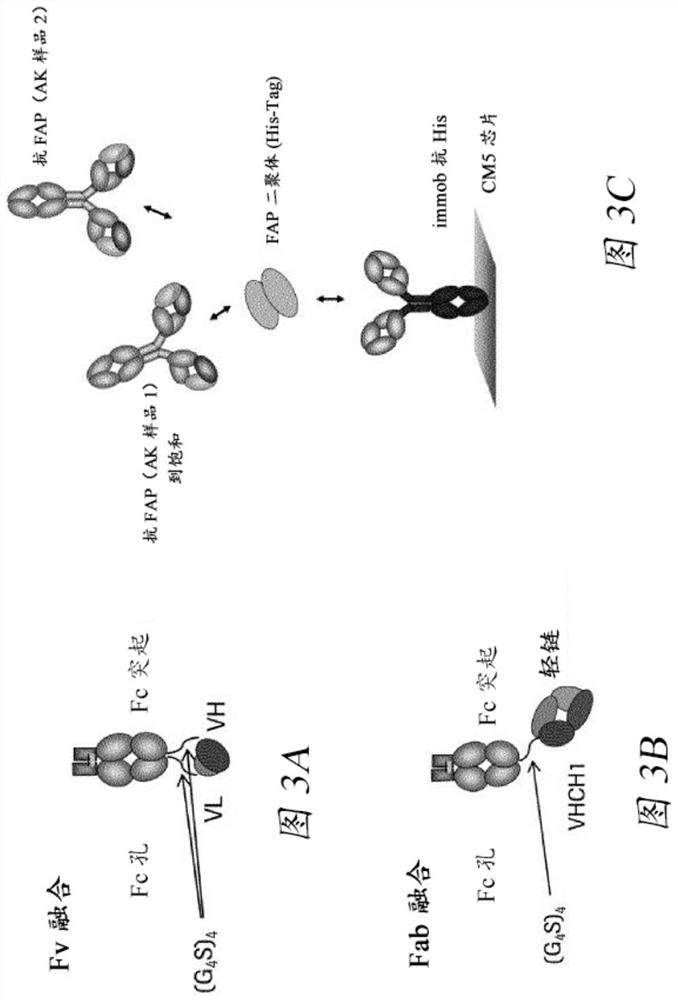
Bispecific antigen binding molecules with trivalent binding to cd40
An antigen-binding molecule and bispecific technology, which is applied in the fields of antibody medical components, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, medical preparations containing active ingredients, etc., can solve the problem of insufficient pathway activation, Issues such as narrow therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0523] The following are examples of methods and compositions of the present invention. It should be understood that various other embodiments may be practiced given the general description provided above.
[0524] recombinant DNA technology
[0525]Standard methods were used to manipulate DNA, as described in Sambrook et al., Molecular cloning: A laboratorymanual; Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989. Molecular biology reagents were used according to the manufacturer's instructions. General information on the nucleotide sequences of human immunoglobulin light and heavy chains is given in the following reference: Kabat, E.A. et al., (1991) Sequences of Proteins of Immunological Interest, Fifth Edition, NIH Publication No 91- 3242.
[0526] DNA sequencing
[0527] DNA sequence was determined by double-stranded sequencing.
[0528] gene synthesis
[0529] The desired gene segments were generated by PCR using appropriate templates, or synt...
example 1
[0542] Generation of new antibodies against fibroblast activation protein (FAP)
[0543] 1.1 Immunization of mice
[0544] Balb / c and NMRI mice were used for immunization. Animals were housed in an AAALACi accredited animal facility in accordance with Appendix A "Guidelines for the Accommodation and Care of Animals". All animal immunization protocols and experiments were approved by the Government of Upper Bavaria (license number 55.2-1-54-2531-19-10) and were performed in accordance with German Animal Welfare Act and European Parliament and Council Directive 2010 / 63. 6-8 week old Balb / c and NMRI mice (n=5) received human fibroblast activation protein alpha (amino acids 27-759; accession number NP_004451) covalently linked to a His-tag (SEQ ID NO:93) Four rounds of immunizations with the extracellular domain. Mice were anesthetized with a mixture of oxygen and isoflurane prior to each immunization. For the first immunization, 30 μg protein in PBS pH 7.4 was mixed with an e...
example 2
[0620] Generation and Production of Humanized Variants of Anti-CD40 Antibody S2C6
[0621] 2.1 Generation of humanized variants of anti-CD40 antibody S2C6
[0622] 2.2.1 Methods
[0623]During the humanization of the anti-CD40 binder S2C6 comprising the VH of SEQ ID NO:51 and the VL of SEQ ID NO:52, in order to identify a suitable human acceptor framework, a combination of two methods was used. On the one hand, classical approaches are employed by finding an acceptor framework with high sequence homology, grafting CDRs onto this framework, and assessing which backmutations can be envisaged. More specifically, the impact of each amino acid difference of the identified framework from the parent antibody is judged on the structural integrity of the binding agent and, where appropriate, backmutations towards the parent sequence are introduced. Structural assessments were based on Fv region homology models of the parental antibody and its humanized version, created with an in-hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com